Mixed water system ionic battery and application thereof
An ion battery, water system technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of short-circuit batteries, low energy density, crystallization, etc., to solve the problem of short cycle life, high cycle stability, and simple manufacturing process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] In a beaker, weigh 78g of sodium carbonate, 1000g of tetrabutyl titanate, 508g of phosphoric acid and 50g of acetylene black, and disperse them in 5L of ethanol, ball mill and stir at 50Hz for 1h, and dry at 90°C to obtain the precursor; then dry The precursor was transferred from the beaker to an agate mortar and ground for half an hour, and then the uniformly ground powder sample was transferred to a 10L capacity nitrogen atmosphere reaction furnace, and the temperature in the reaction furnace was raised at a rate of 5°C / min. When the temperature When the temperature is raised to 400°C, the temperature is kept constant for 5 hours, then the temperature is raised to 800°C at 5°C / min, the temperature is kept constant for 10 hours, and finally cooled to room temperature naturally to obtain carbon composite NaTi 2 (PO 4 ) 3 Material.
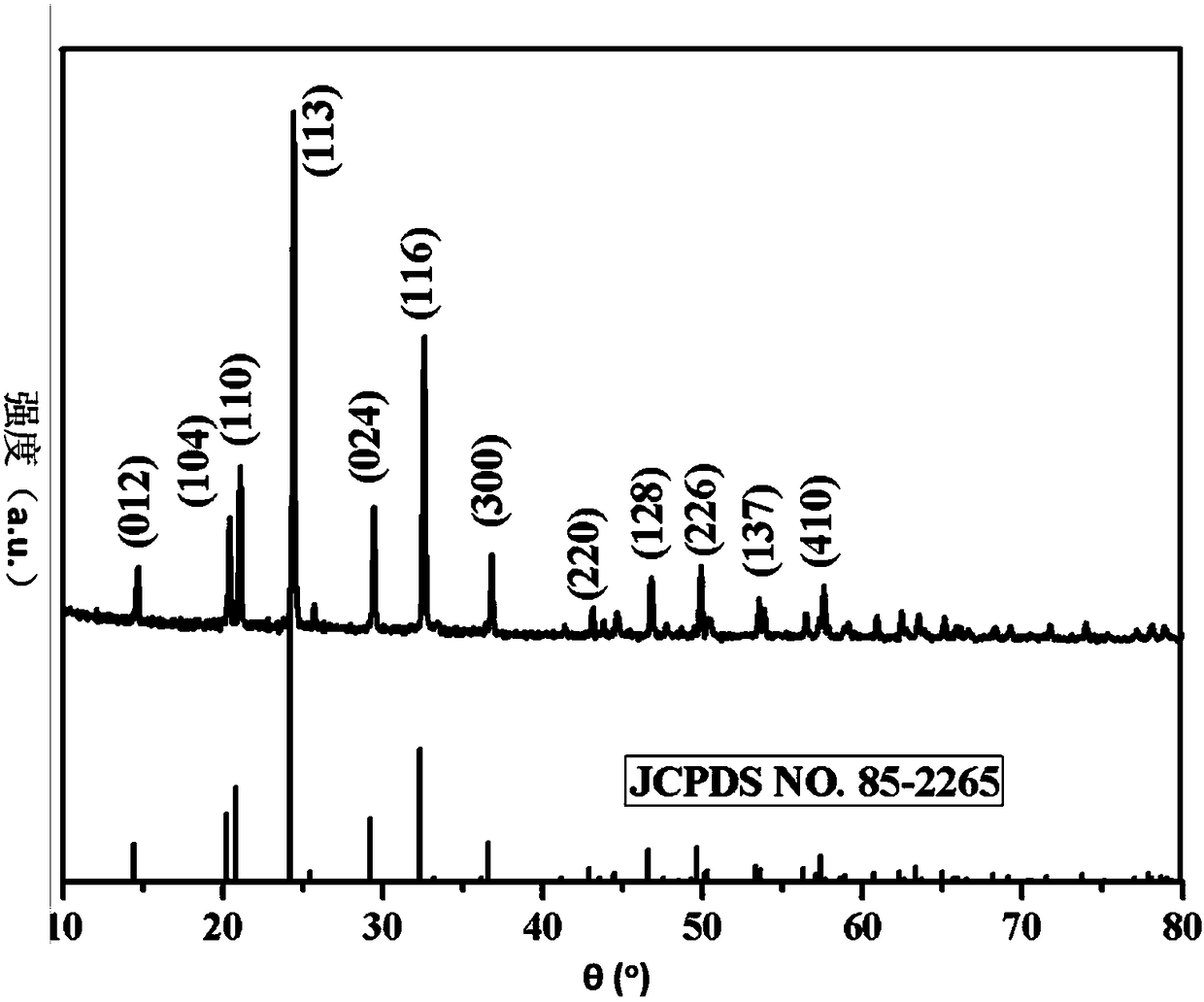
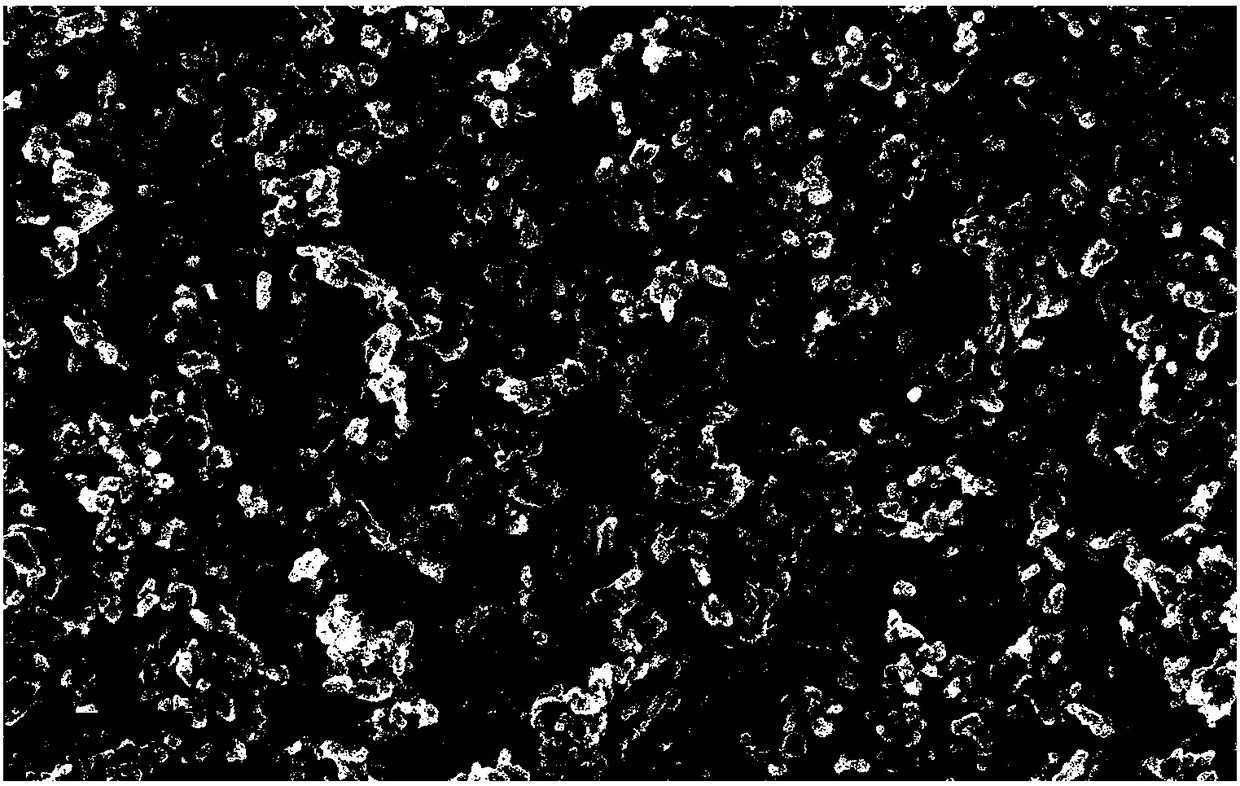
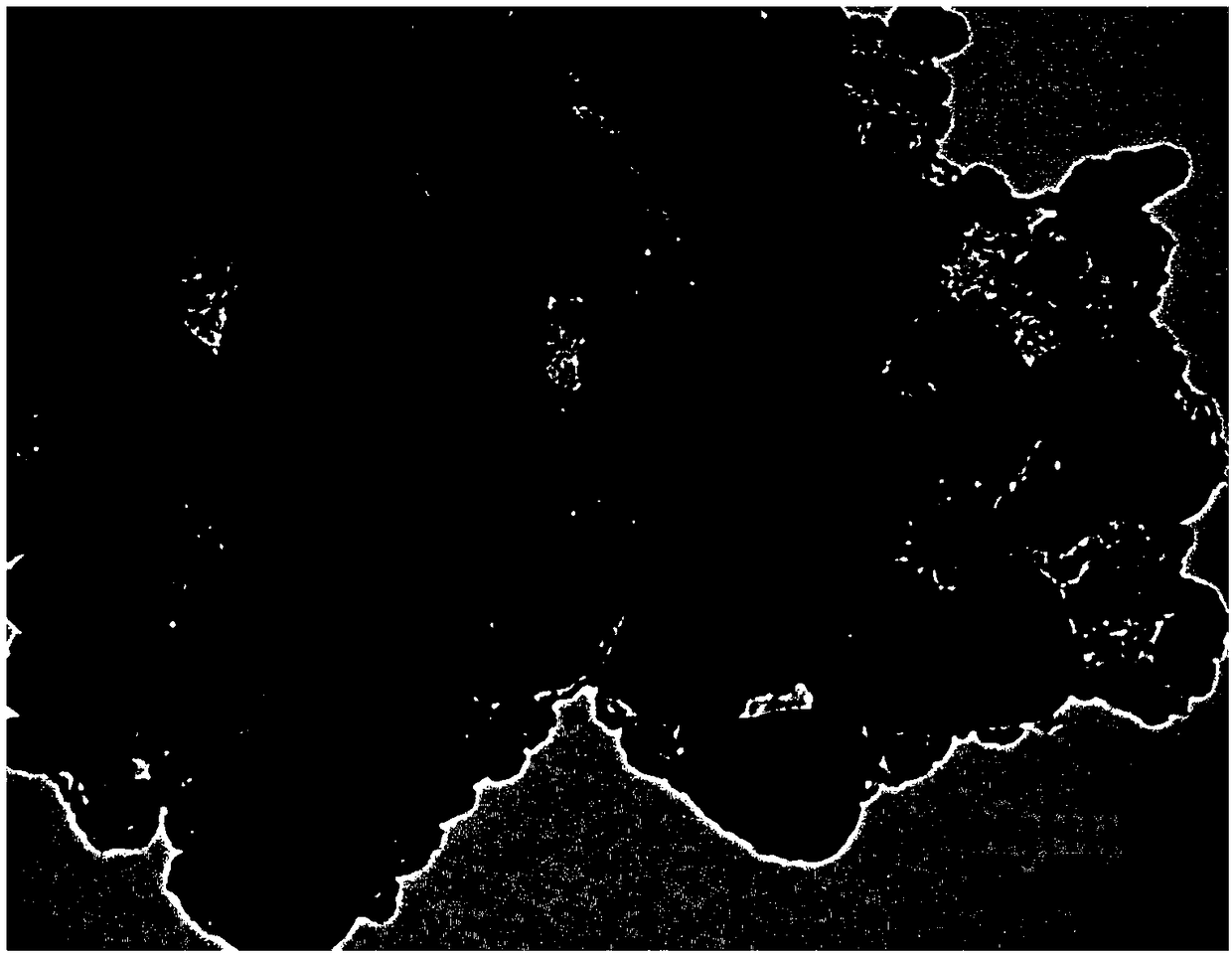
[0066] Adopt X-ray powder diffractometer to the carbon composite NaTi that present embodiment 1 obtains 2 (PO 4 ) 3 The material was ...
Embodiment 2
[0071] In a beaker, weigh 36g of disodium edetate, 500g of titanium dioxide and 508g of phosphoric acid, and disperse them in 5L of methanol, then add 50g of graphite, stir by ball milling at 50Hz for 1h, and dry at 85°C to obtain the precursor; Then transfer the dry precursor from the beaker to an agate mortar and grind for half an hour, then transfer the uniformly ground powder sample to a 10L capacity argon atmosphere reaction furnace, and place the reaction furnace at a rate of 6°C / min Raise the temperature, when the temperature rises to 500°C, keep the temperature constant for 5 hours, then raise the temperature to 850°C at 6°C / min, keep the temperature constant for 10 hours, and finally cool down to room temperature naturally to obtain carbon-composite NaTi 2 (PO 4 ) 3 Material.
[0072] Similar to Example 1, X-ray diffraction analysis, scanning electron microscope analysis, transmission electron microscope analysis and Raman spectroscopic analysis (figure not shown) a...
Embodiment 3
[0074] In a beaker, weigh 50g of disodium phosphate, 800g of metatitanic acid, 508g of phosphoric acid and 50g of Ketjen black, and disperse them in 5L of isopropanol, stir by ball milling at 50Hz for 1h, and dry at 95°C to obtain the precursor; then Transfer the dry precursor from the beaker to an agate mortar and grind for half an hour, then transfer the uniformly ground powder sample to a 10L capacity hydrogen atmosphere reaction furnace, and raise the temperature in the reaction furnace at a rate of 5°C / min. When the temperature was raised to 450°C, the temperature was kept constant for 5 hours, then the temperature was raised to 900°C at 6°C / min, the temperature was kept constant for 10 hours, and finally cooled naturally to room temperature to obtain carbon-composite NaTi 2 (PO 4 ) 3 Material.
[0075] Similar to Example 1, X-ray diffraction analysis, scanning electron microscope analysis, transmission electron microscope analysis and Raman spectroscopic analysis (figu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| First discharge capacity | aaaaa | aaaaa |
| Energy density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com