Sulfo group polymer electrolyte, as well as in-situ preparation method and application thereof
An in-situ preparation and polymer technology, applied in the field of electrochemistry, can solve the problems of hidden danger of charging, high reactivity, easy volatility, etc., and achieve the effect of satisfying high-current charging and discharging, improving safety performance, and improving thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
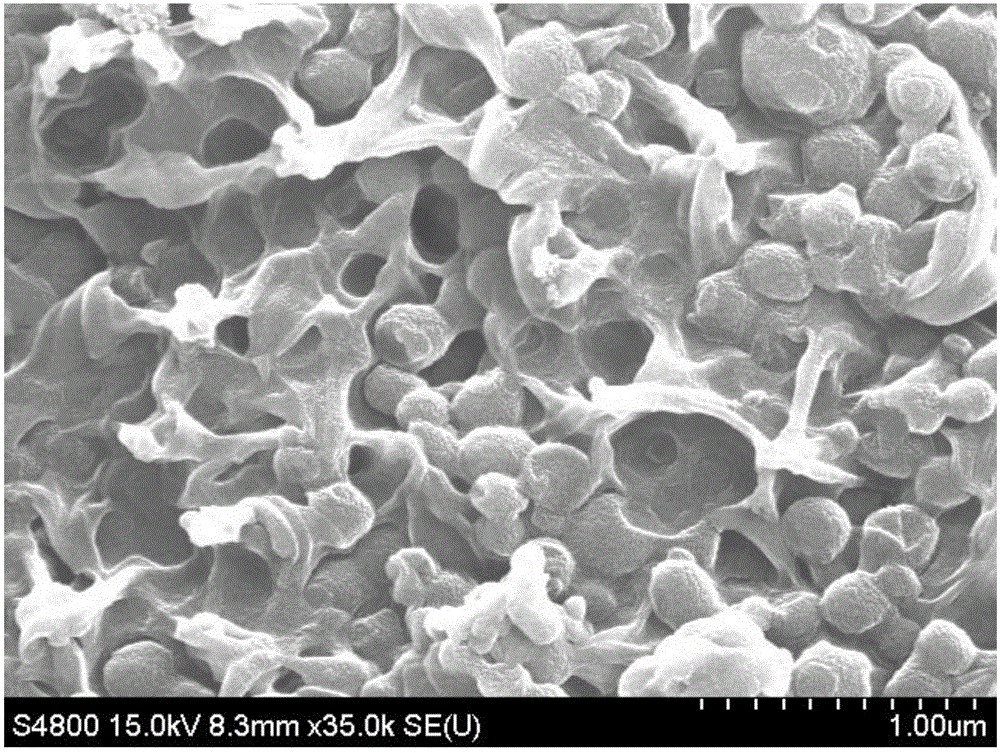
[0030] Dissolve 5g of polyvinylidene fluoride powder in 45g of N-methylpyrrolidone, after completely dissolving, add 5g of 3-mercaptopropyltrimethoxysilane, stir to make it evenly mixed, take 0.5mL and spread it on 25.4mm× Place on a 76.2mm glass slide and dry in a vacuum oven at 80°C for 12h. That is, a polymer film containing -SH groups is obtained.
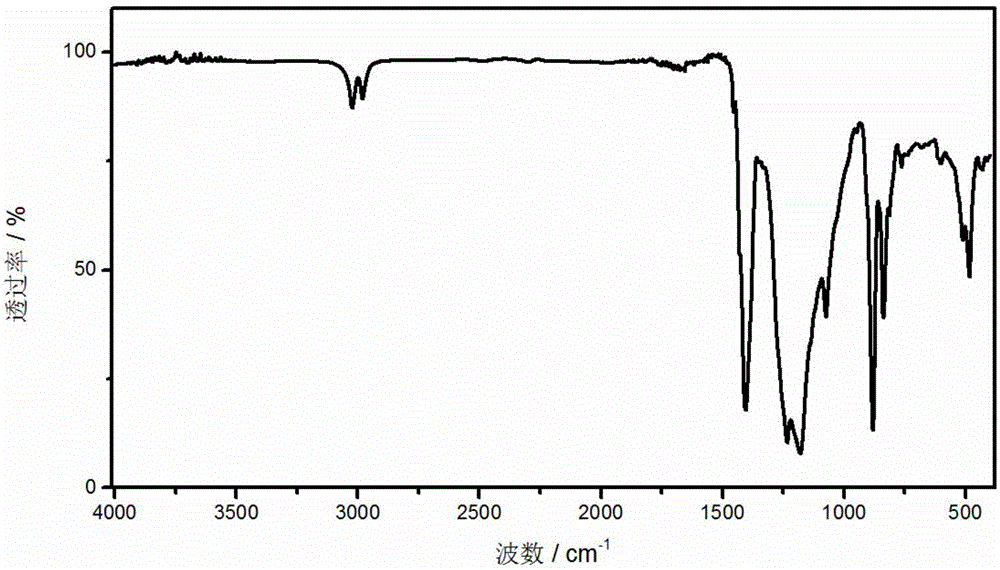
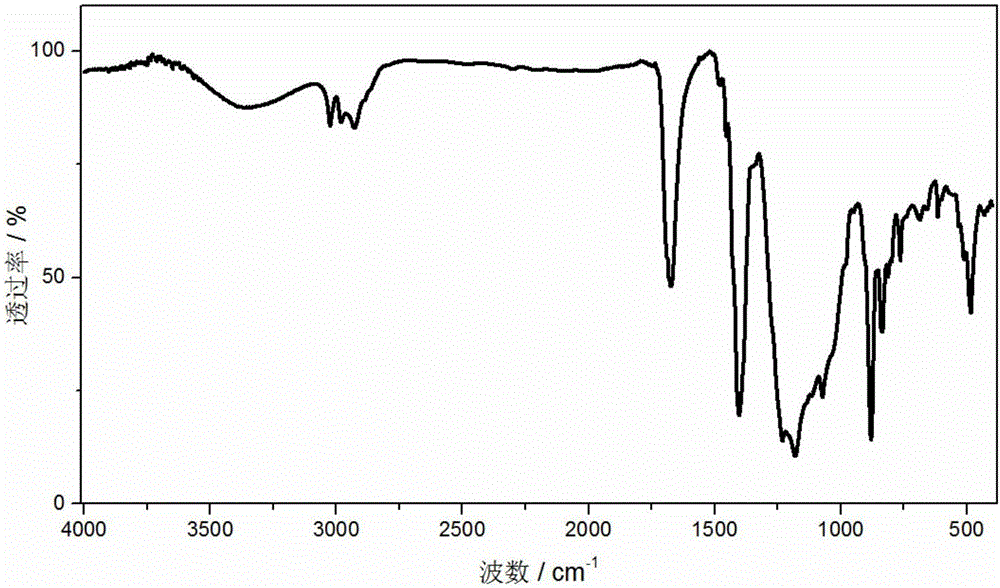
[0031] The prepared polymer film containing -SH groups was immersed in 35% H 2 o 2 In the solution, oxidize at 65°C for 6h while hydrolyzing the silane to obtain a surface containing -SO 3 HSiO2 For the inorganic-organic composite polymer membrane, take out the composite polymer membrane and wash it three times with deionized water. The pretreated composite polymer membrane was then placed in 0.2M H 2 SO 4 The solution was acidified for 1 hour, rinsed with deionized water three times, and then the membrane was put into water and boiled for 1 hour. The acidified composite polymer membrane was then placed in 1M CH 3 Lithiu...
Embodiment 2
[0033] Dissolve 5g of polyvinylidene fluoride-hexafluoropropylene copolymer powder in 45g of N-methylpyrrolidone, after completely dissolving, add 5g of 3-mercaptopropyltrimethoxysilane, and simultaneously add 2g of 10% HNO 3 , to promote the hydrolysis of 3-mercaptopropyltrimethoxysilane, and at the same time play the role of preliminary oxidation. After stirring to make it fully uniform, take 0.5mL and spread it on a 25.4mm×76.2mm glass slide. 1. Under the condition of relative humidity of 50% RH, dry in a vacuum oven at 80° C. for 12 hours. That is, a polymer film containing -SH groups is obtained.
[0034] The prepared polymer film containing -SH groups was immersed in 35% H 2 o 2 In the solution, oxidize at 65°C for 6h while hydrolyzing the silane to obtain a surface containing -SO 3 H SiO 2 For the inorganic-organic composite polymer membrane, take out the composite polymer membrane and wash it three times with deionized water. The pretreated composite polymer membr...
Embodiment 3
[0038] Dissolve 5g of polyvinylidene fluoride powder in 45g of N-methylpyrrolidone, after completely dissolving, add 5g of 3-mercaptopropyltrimethoxysilane, add 1g of 100nm aluminum oxide, stir to make it fully uniform, then take 0.5mL was coated on a 25.4mm×76.2mm glass slide, and dried in a vacuum oven at 80°C for 12h. That is, a polymer film containing -SH groups is obtained.
[0039] The prepared polymer film containing -SH groups was immersed in 35% H 2 o 2 In the solution, oxidize at 65°C for 6h while hydrolyzing the silane to obtain a surface containing -SO 3 H SiO 2 For the inorganic-organic composite polymer membrane, take out the composite polymer membrane and wash it three times with deionized water. The pretreated composite polymer membrane was then placed in 0.2M H 2 SO 4 The solution was acidified for 1 hour, rinsed with deionized water three times, and then the membrane was put into water and boiled for 1 hour. The acidified composite polymer membrane was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com