Method for preparing lithium carbonate through two-stage conversion of lithium ore
A lithium ore and lithium carbonate technology, applied in the direction of lithium carbonate;/acid carbonate, etc., can solve the problems of low lithium leaching rate, difficult decomposition of lithium ore, and low recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
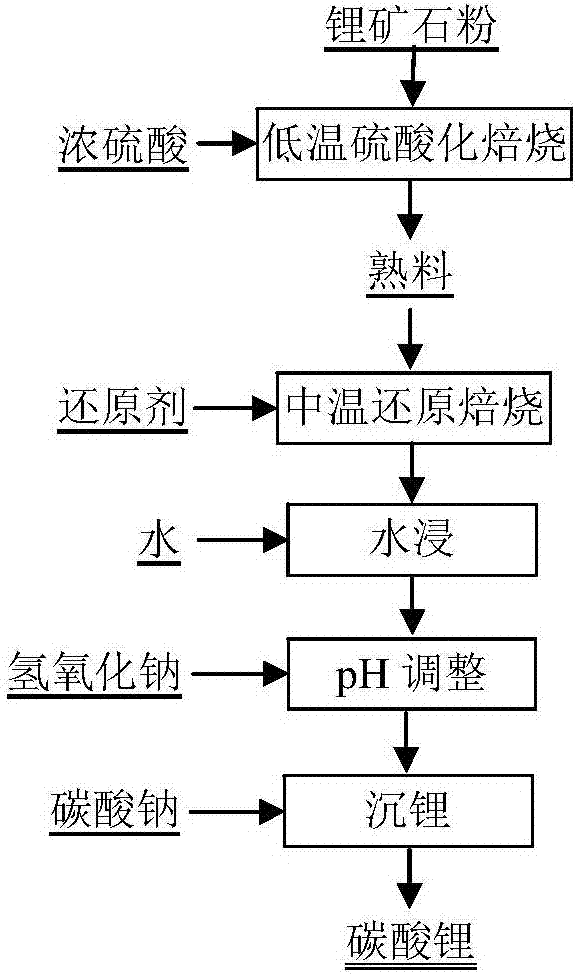
[0033] Mix 100g of mixed lithium concentrate powder with 95% concentrated sulfuric acid to obtain a mixture. The amount of concentrated sulfuric acid added is 1.5 times the theoretical amount of sulfuric acid required. The mixture is aged and roasted at 300°C for 2 hours, and the obtained clinker is crushed. Mix it with coal powder, and then reduce and roast at 900°C for 30 minutes. The amount of coal powder added is twice the amount of theoretical coal powder required for the complete reduction and decomposition of aluminum sulfate and iron sulfate in the clinker; The amount is added according to the leaching solid-liquid mass ratio of 1:3, the leaching temperature is 50°C, and the leaching time is 30 minutes, then filtered to obtain a lithium-containing leaching solution, the pH value of the leaching solution is adjusted to 11 with sodium hydroxide, and filtering is obtained. The obtained filtrate is precipitated with sodium carbonate to obtain lithium carbonate.
Embodiment 2
[0035]Take 100g lepidolite ore powder and mix with 90% concentrated sulfuric acid to obtain a mixture. The amount of concentrated sulfuric acid added is twice the theoretical amount of sulfuric acid required. The mixture is matured and roasted at 250°C for 2 hours. The obtained matured material is crushed and mixed with Coal powder is mixed, and then reduced and roasted at 600°C for 1 hour. The amount of coal powder added is twice the amount of theoretical coal powder required for the complete reduction and decomposition of aluminum sulfate and ferric sulfate in the clinker; Add according to the leaching solid-liquid mass ratio of 1:2, the leaching temperature is 90°C, the leaching time is 30min, and then filter to obtain the lithium-containing leaching solution; adjust the pH value of the leaching solution to 11 with sodium hydroxide, filter, and the obtained filtrate is precipitated with sodium carbonate to obtain carbonic acid lithium.
Embodiment 3
[0037] Mix 100g of spodumene concentrate powder with 95% concentrated sulfuric acid to obtain a mixture. The amount of concentrated sulfuric acid added is twice the theoretical amount of sulfuric acid required. The mixture is matured and roasted at 250°C for 2 hours, and the obtained matured material is crushed After that, it is mixed with coal powder, and then reduced and roasted at 600°C for 1 hour. The amount of coal powder added is twice the amount of theoretical coal powder required for the complete reduction and decomposition of aluminum sulfate and iron sulfate in the clinker; The amount to be added is added according to the leaching solid-to-liquid mass ratio of 1:3, the leaching temperature is 50°C, and the leaching time is 30 minutes, and then filtered to obtain a lithium-containing leaching solution; the pH value of the leaching solution is adjusted to 11 with sodium hydroxide, and filtered, and the obtained filtrate is precipitated with sodium carbonate Lithium carb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com