Lithium carbonate production technology
A production process, lithium carbonate technology, applied in the direction of lithium carbonate;/acid carbonate, etc., can solve the problems of poor control of impurity acid in the product, production method has no practical value, and the product cannot meet the industry standard, etc. , to achieve the effects of easy control of the reaction process, improvement of market competitiveness, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
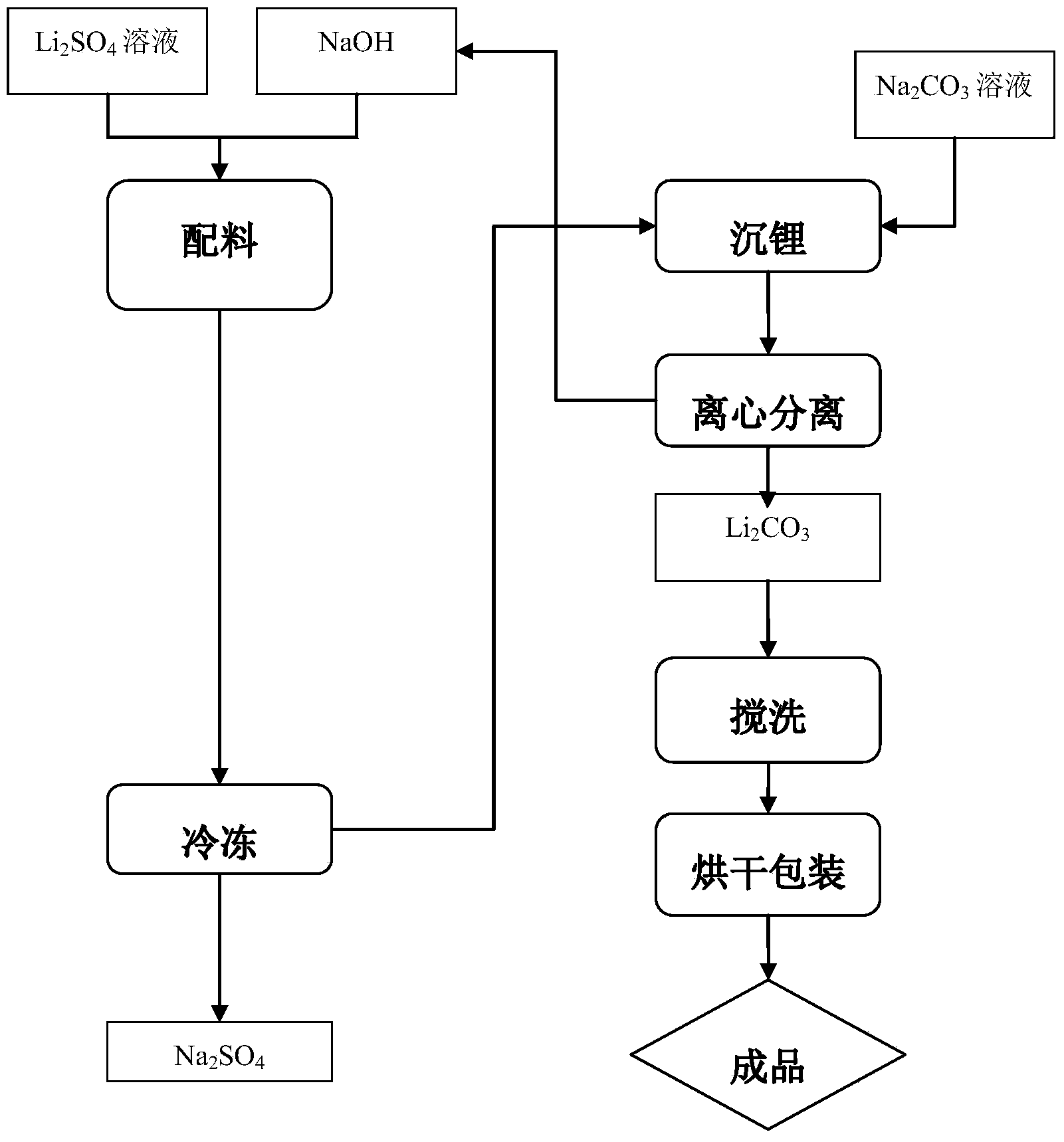
[0024] Ingredients and its freezing: Will Li 2 Li with an O concentration of 50 g / L 2 SO 4 Solution 3m 3 , the mass fraction is 30% NaOH solution 1.3~1.4m 3 mix, gain 2 SO 4 and NaOH mixed solution. Freeze the mixed solution at -2°C to precipitate Na 2 SO 4 After the crystals are separated, LiOH solution is obtained, and the Li of LiOH solution 2 O concentration is 58g / L.
[0025] Lithium sinking: 1.5m 3 A saturated solution of sodium carbonate with a concentration of 285g / L was heated to 90°C, and 2m 3 LiOH solution, the reaction produces Li 2 CO 3 Liquid-solid mixture of precipitate and NaOH solution.
[0026] Will Li 2 CO 3 The liquid-solid mixture of the precipitate and NaOH solution was centrifuged to obtain Li 2 CO 3 Crude and Li 2 CO 3 Crude product, washed with water to remove Li 2 CO 3 Soluble impurity ions in the crude product to obtain purified Li 2 CO 3 Wet product; Will Li 2 CO 3 After the wet product is dried, lithium carbonate 261Kg is o...
Embodiment 2
[0028] Ingredients and its freezing: Will Li 2 The concentration of O is 50g / L of Li 2 SO 4 Solution 3m 3 , the mass fraction is 30% NaOH solution 1.3~1.4m 3 mix, gain 2 SO 4 and NaOH mixed solution. Freeze the mixed solution at -5°C to precipitate Na 2 SO 4 After the crystals are separated, LiOH solution is obtained, and the Li in LiOH solution 2 O concentration is 58g / L.
[0029] Lithium sinking: 1.5m 3 The saturated solution of sodium carbonate with a concentration of 285g / L was heated to 95°C, and 2.3m 3 LiOH solution, get the reaction to generate Li 2 CO 3 Liquid-solid mixture of precipitate and NaOH solution.
[0030] Will Li 2 CO 3 After centrifugation of the liquid-solid mixture of the precipitate and NaOH solution, the Li 2 CO 3 Soluble impurity ions in the crude product to obtain purified Li 2 CO 3 Wet product; Will Li 2 CO 3 After the wet product is dried, lithium carbonate 278Kg is obtained. The indicators of lithium carbonate are as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com