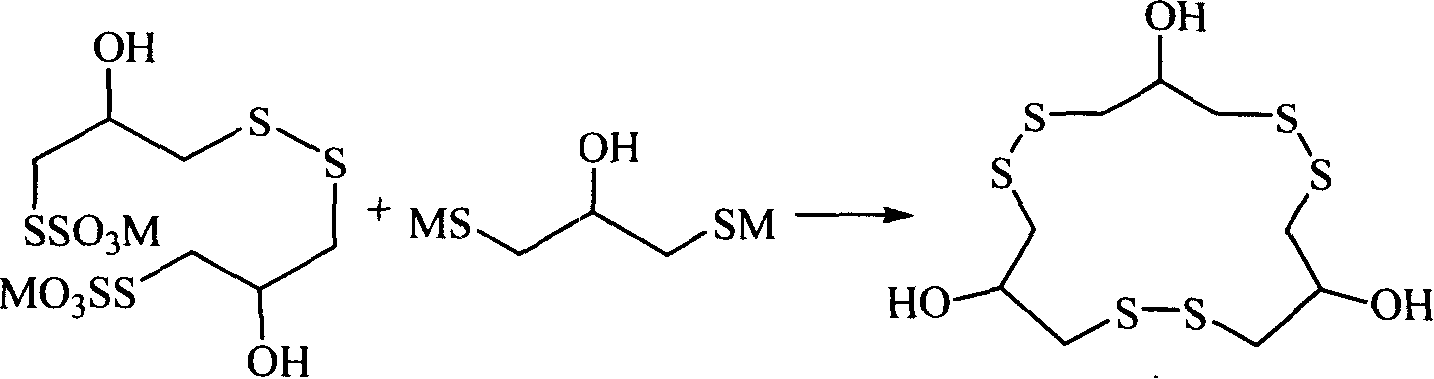
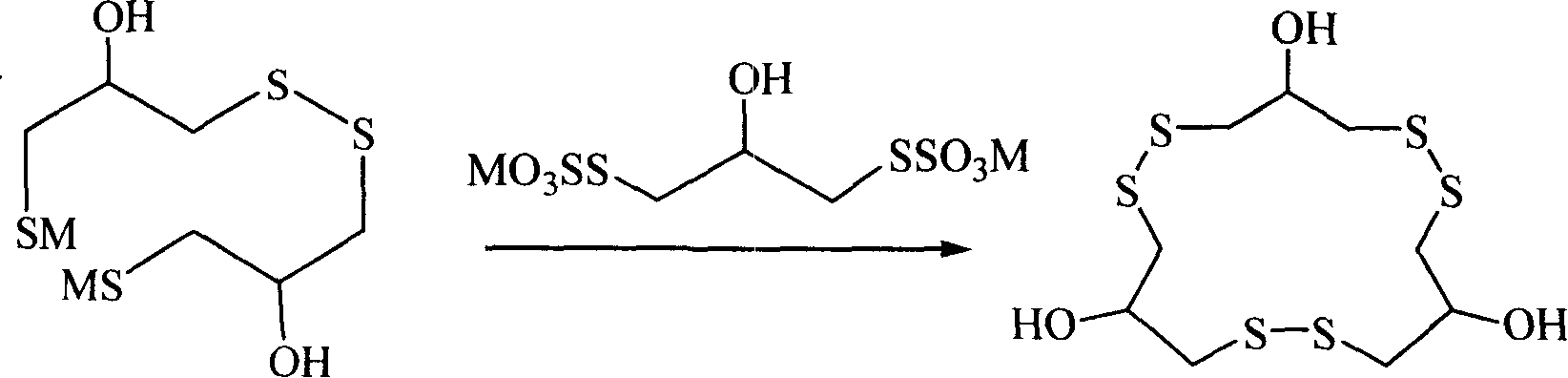
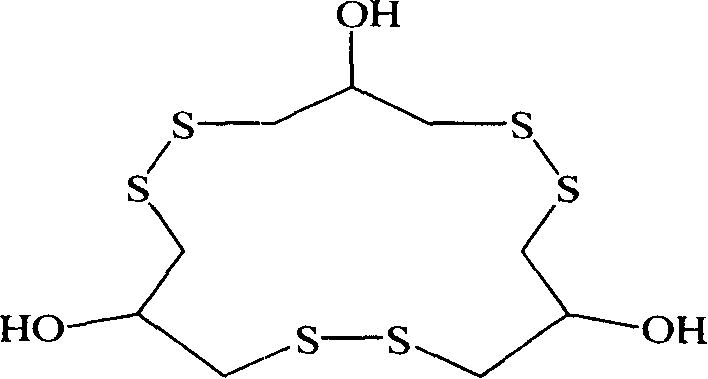
Method for synthesizing macrocyclic polydithioether compound gymnorrhizol
A technology of cyclohexathiol and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of poor reaction reproducibility and low yield, and achieve the effects of good reproducibility, high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Synthesis of 3,3'-dithiobis(1-chloro-2-propanol):
[0055] Add 3 grams of sodium borohydride and 7.7 grams of sulfur powder into a three-necked flask with nitrogen gas, reflux condenser and drying tube, cool in an ice bath to below 5°C, and slowly add 40 milliliters of anhydrous tetrahydrofuran dropwise, and the material gradually turns red. , and gas was released, the ice bath was removed after the addition, and the stirring was continued for 30 minutes, and a yellow solid was formed. Slowly add 3.7 g of epichlorohydrin dissolved in 10 ml of anhydrous tetrahydrofuran dropwise to the above reactant, and stir at room temperature for 5 hours; add water to decompose excess sodium trithioborohydride, separate the organic layer, and use diethyl ether for the aqueous phase Extract, combine the extracts, dry over anhydrous magnesium sulfate, filter and evaporate the solvent under reduced pressure, use petroleum ether: acetone (volume ratio 5:1) as the eluent, and ob...
Embodiment 2
[0057] Example 2: Synthesis of 3,3'-dithiobis(1-chloro-2-propanol):
[0058] Mix 8.2 grams of sodium sulfide nonahydrate and 1.1 grams of sulfur powder, add 40 milliliters of 95% ethanol and heat to reflux, add 4 grams of 1-bromo-3-chloro-2-propanol after 30 minutes, continue to heat and reflux for 3 hours, evaporate under reduced pressure The solvent was removed, the residue was extracted with diethyl ether, the organic phase was dried with sodium sulfate, filtered, and concentrated to obtain a red oil, using petroleum ether: acetone (volume ratio 5:1) as the eluent, the white powder 4.8 was obtained by silica gel column chromatography gram, yield 85%. Since bromine is more reactive than chlorine, a chlorine-only product is obtained.
Embodiment 3
[0059] Example 3: Synthesis of 3,3'-dithiobis(1-chloro-2-propanol):
[0060] Dissolve 2.16 g of 1-bromo-3-chloroisopropanol in 15 ml of 50% ethanol, add 3.1 g of sodium thiosulfate pentahydrate, heat to reflux for 2 hours, add 3.2 g of solid iodine while hot, and continue heating to reflux for 1 hour After cooling down, the reactant was extracted with ethyl acetate, and the combined organic phases were washed successively with sodium bicarbonate solution, sodium thiosulfate solution, saturated brine, dried over magnesium sulfate, concentrated by filtration, and the residue was prepared with petroleum ether: acetone (volume ratio 5:1) was used as the eluent, and 1 g of white solid was obtained by silica gel column chromatography with a yield of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com