Method for producing manganese sulfate from manganese oxide ores
A technology of manganese oxide ore and manganese sulfate, applied in the direction of manganese sulfate, etc., can solve the problems of slow leaching speed, low leaching rate, and high energy consumption, and achieve the effects of low cost, improved product quality, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
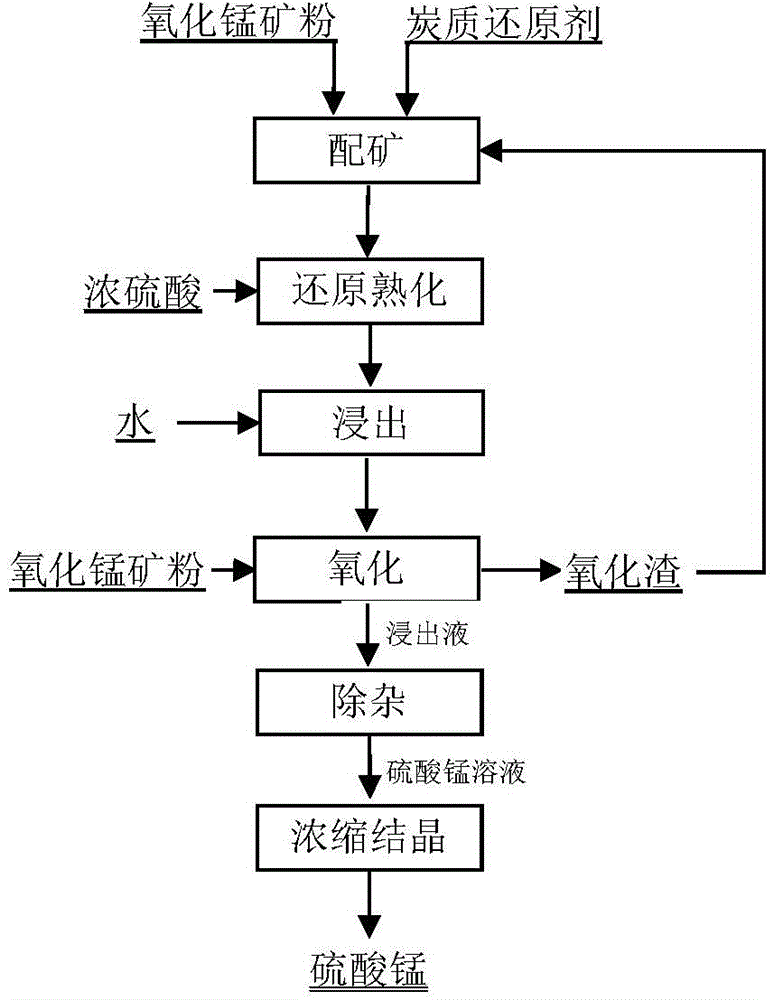
[0038]Take manganese oxide ore powder with a manganese content of 25% and mix it evenly with lignite powder. The amount of lignite powder is added according to the molar ratio of fixed carbon in coal powder to manganese in manganese oxide ore at 1:1, and then add concentrated sulfuric acid with a concentration of 95%. The amount of concentrated sulfuric acid is added according to the molar ratio of sulfuric acid to manganese oxide ore powder of 1.5:1, the initial concentration of sulfuric acid in the mixture is 90%, the reaction is self-heated to the highest temperature of 250°C and matured for 2 hours, and then water is added to the matured Slurry leaching is performed on the raw material, the leaching temperature is 95°C, the leaching time is 60min, and the manganese leaching rate is 97.5%. The leached ore pulp is oxidized with manganese oxide ore powder. The amount of manganese oxide ore powder added is 2.5% of the mass of manganese oxide ore powder during ore blending. Rhod...
Embodiment 2
[0040] Take manganese oxide ore powder with a manganese content of 40% and mix it evenly with lignite powder. The amount of lignite powder added is formulated according to the molar ratio of fixed carbon in coal powder to manganese in manganese oxide ore at 1:1.5, and then add concentrated sulfuric acid with a concentration of 95%. , the amount of concentrated sulfuric acid is added according to the molar ratio of sulfuric acid to manganese oxide ore powder 1.2:1, the reaction is self-heated to a maximum temperature of 250°C and matured for 2 hours, and then water is added to slurry and leach the matured material at a leaching temperature of 65°C , leaching time 60min, manganese leaching rate 98.5%. The leached ore pulp is filtered to obtain the leachate, and the leachate is oxidized with manganese oxide ore powder. The amount of manganese oxide ore powder added is 10% of the mass of manganese oxide ore powder during ore blending, and then filtered to obtain oxide residue and m...
Embodiment 3
[0042] Get the manganese oxide ore powder that manganese content is 40% and the oxidized slag of embodiment 2 and brown coal powder mix evenly, the add-on of brown coal powder is mixed into by the mol ratio 1:1.5 of manganese in the fixed carbon in the coal powder and manganese oxide ore, then add Concentrated sulfuric acid with a concentration of 95%, the amount of concentrated sulfuric acid is added according to the molar ratio of sulfuric acid to manganese oxide ore powder 1.2:1, the initial concentration of sulfuric acid in the mixture is 80%, and the reaction is self-heated to the highest temperature of 200°C and then matured for 2 hours , and then add water to carry out slurry leaching of the mature material, the leaching temperature is 65°C, the leaching time is 60min, and the manganese leaching rate is 98%. The leached ore pulp is oxidized with manganese oxide ore powder, and rhodochrosite powder is used as a neutralizing agent to carry out oxidation neutralization and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com