Processing of cobaltous sulphate/dithionate liquors derived from cobalt resource
A technology of sodium dithionite and liquid agent, which is applied in the field of recycling water and sulfate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
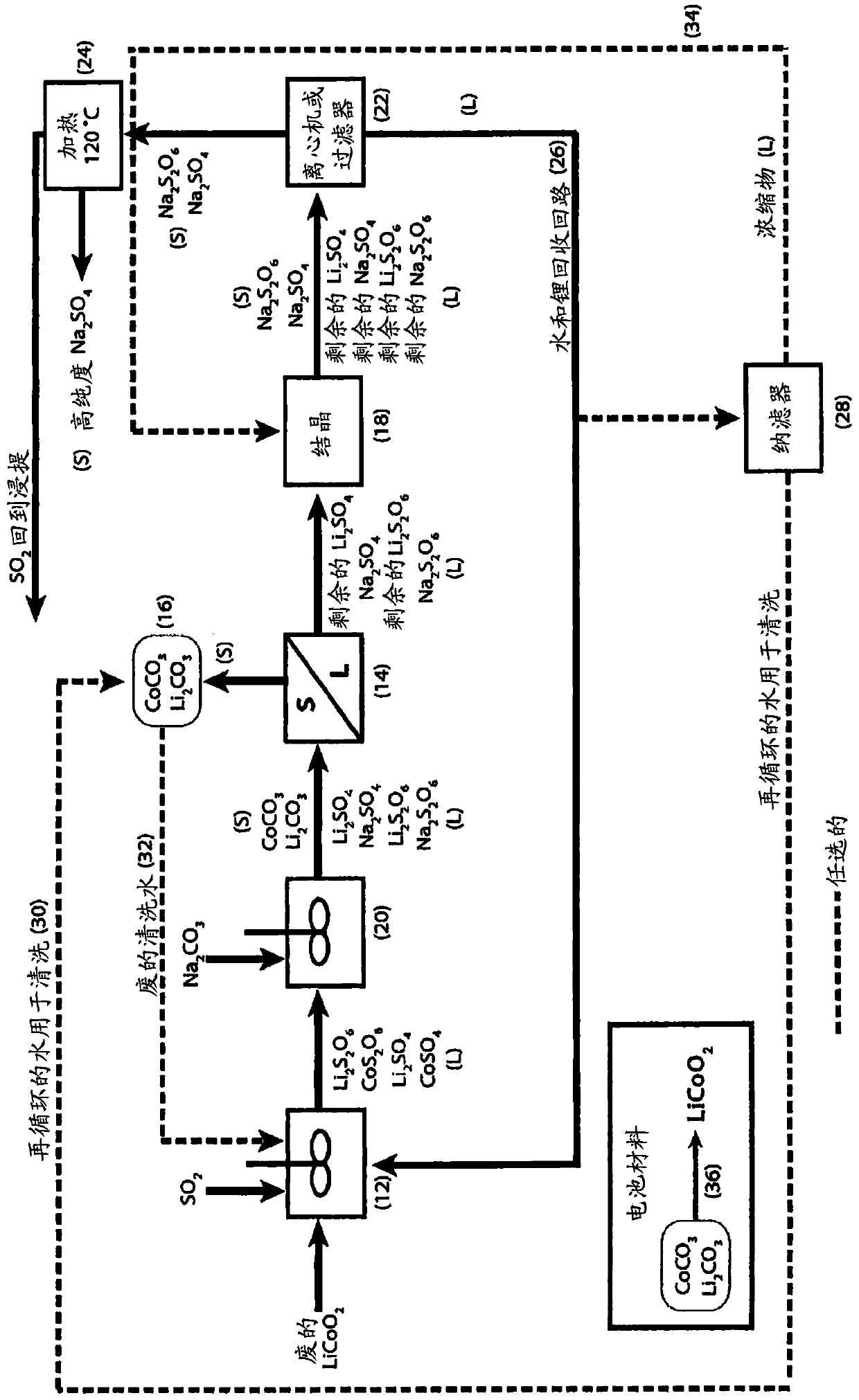
[0035] For Embodiment 1, refer to Figure 1-3 .
[0036] The liquor comprising cobalt sulfate and cobalt dithionate is treated with sodium carbonate to form cobalt carbonate solid and a liquor comprising sodium sulfate and sodium dithionate.
[0037] Lithium sulfate and lithium dithionate, if present, are partially precipitated with cobalt carbonate solid as lithium carbonate solid.
[0038] Solids comprising cobalt carbonate and lithium carbonate solids, if present, are removed from the carbonated liquor by filtration or centrifugation.
[0039] The solids comprising cobalt carbonate and lithium carbonate solids (if present) are washed to remove soluble impurities and to produce clean material for reuse, for example, as a positive electrode material for lithium-ion batteries.
[0040] The filtrate or centrifugate containing sodium sulfate and sodium dithionate together with the remaining lithium sulfate and lithium dithionate (if present) is treated with a crystallizer to c...
Embodiment approach 2
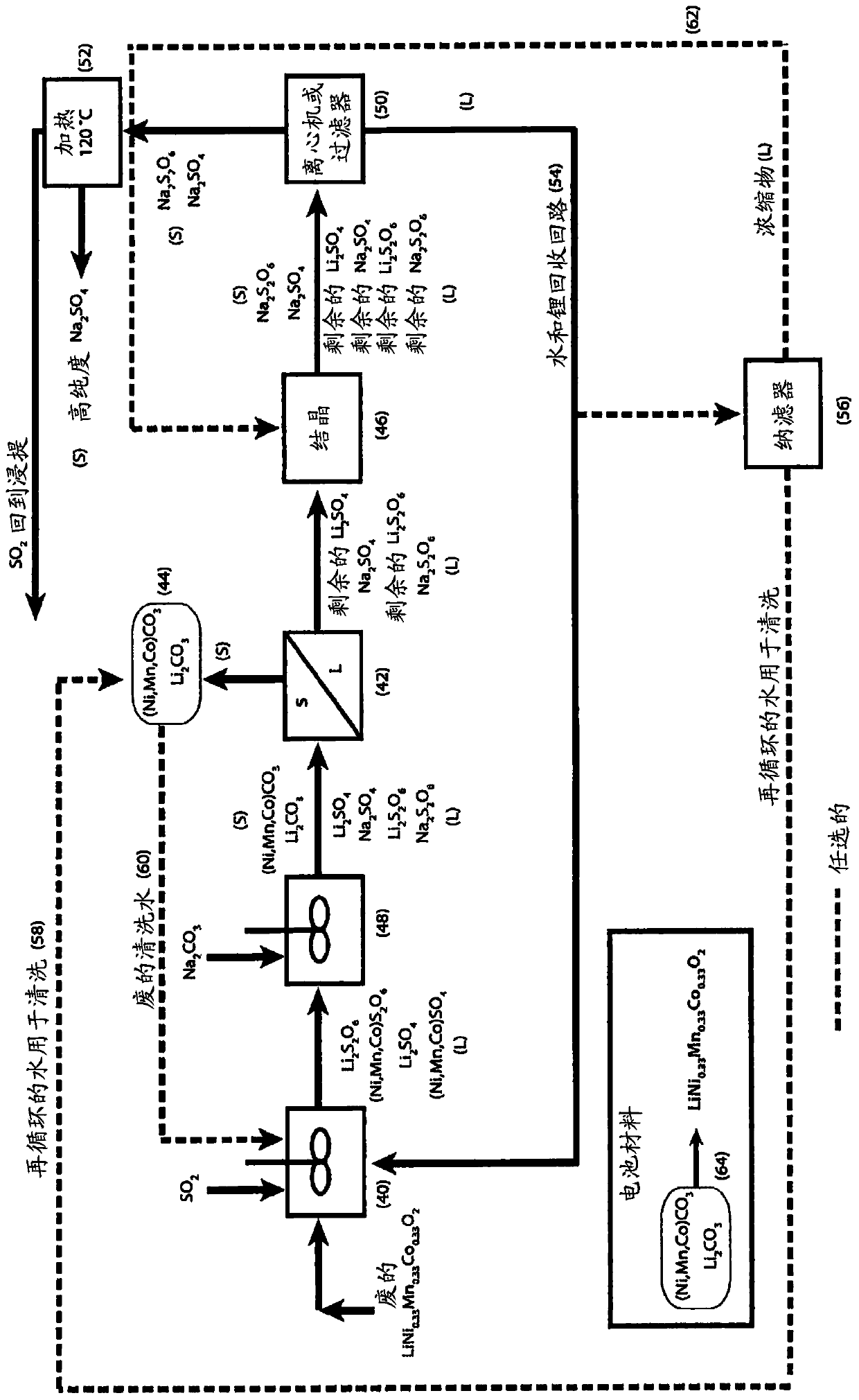
[0047] For the second embodiment, refer to Figure 4-6
[0048] treating a liquor comprising cobalt sulfate and cobalt dithionate with sodium hydroxide to form cobalt hydroxide solid and a liquor comprising sodium sulfate and sodium dithionate;
[0049] removing solids comprising cobalt hydroxide from the hydroxide-treated liquor by filtration or centrifugation;
[0050] Washing solids comprising cobalt hydroxide to remove soluble impurities and produce clean material for reuse, for example, as cathode material for lithium-ion batteries;
[0051] The addition of sodium carbonate to the remaining solution causes the partial precipitation of lithium (if present) as lithium carbonate;
[0052] Lithium carbonate solids are removed from the carbonated liquor by filtration or centrifugation;
[0053] Washing lithium carbonate solids to remove soluble impurities and produce clean material for reuse, such as cathode material for lithium-ion batteries;
[0054] treating the filtrat...
Embodiment approach 3
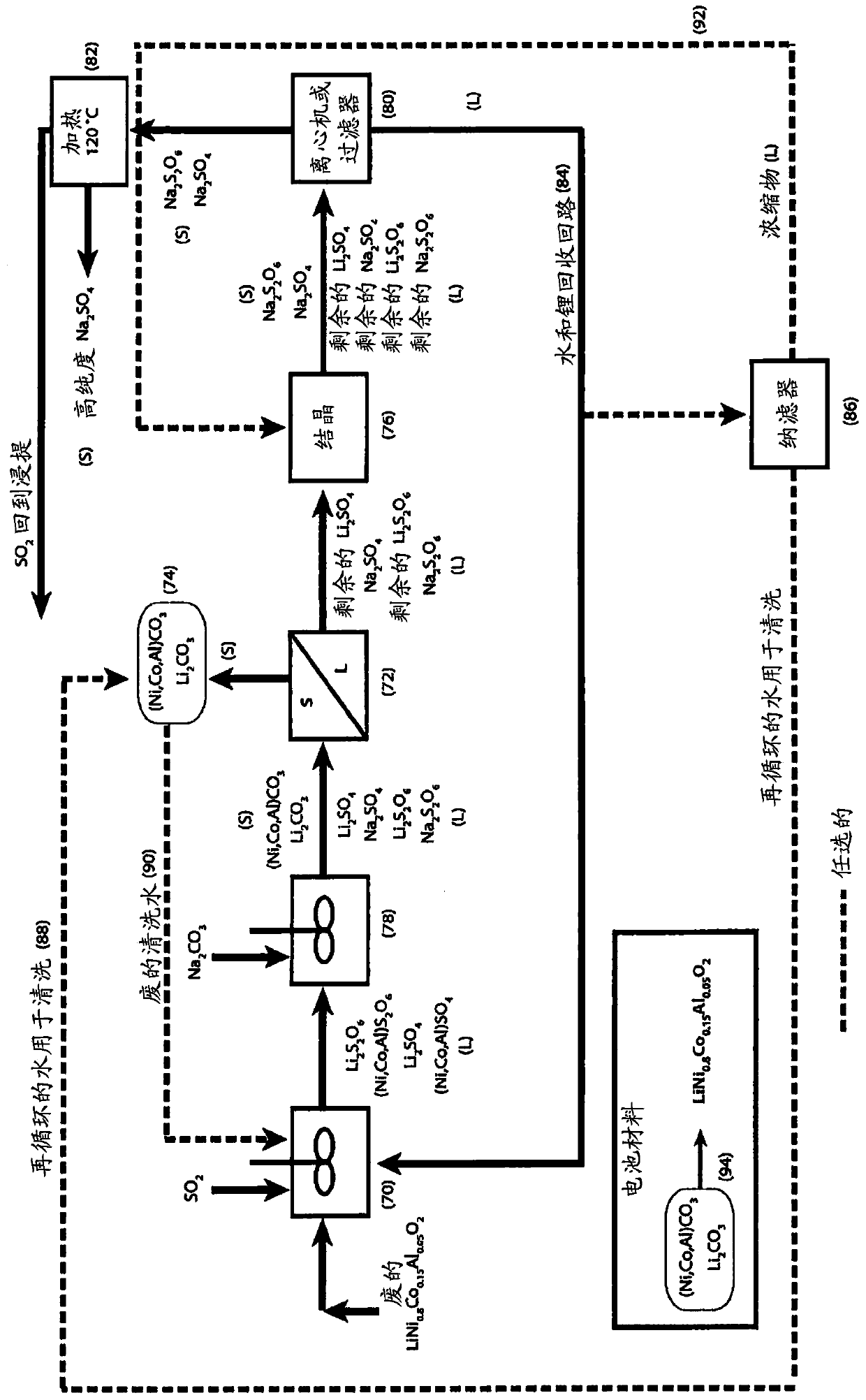
[0059] For the third embodiment, refer to Figure 7-9 .
[0060] treating a liquor comprising cobalt sulfate and cobalt dithionate with lithium hydroxide to form cobalt hydroxide solid and a liquor comprising lithium sulfate and lithium dithionate;
[0061] The lithium hydroxide may be produced by processing lithium carbonate recovered by previous operations of the process. Wietelmann et al. ("Lithium and Lithium Compounds", Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co, 2013, p. 24) describe the production of lithium hydroxide by reacting lithium carbonate with calcium hydroxide;
[0062] removing solids comprising cobalt hydroxide from the hydroxide-treated liquor by filtration or centrifugation;
[0063] Washing solids comprising cobalt hydroxide to remove soluble impurities and produce clean material for reuse, for example, as cathode material for lithium-ion batteries;
[0064] The addition of sodium carbonate to the remaining solution caus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com