Processing of cobaltous sulphate/dithionate liquors derived from cobalt resource
A technology of cobalt hydrosulfate and sodium hydrosulfate, applied in the directions of thiosulfate/dithionite/polythionite, alkali metal sulfite/sulfite, cobalt oxide/cobalt hydroxide, etc. , which can solve problems such as incomprehensible
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
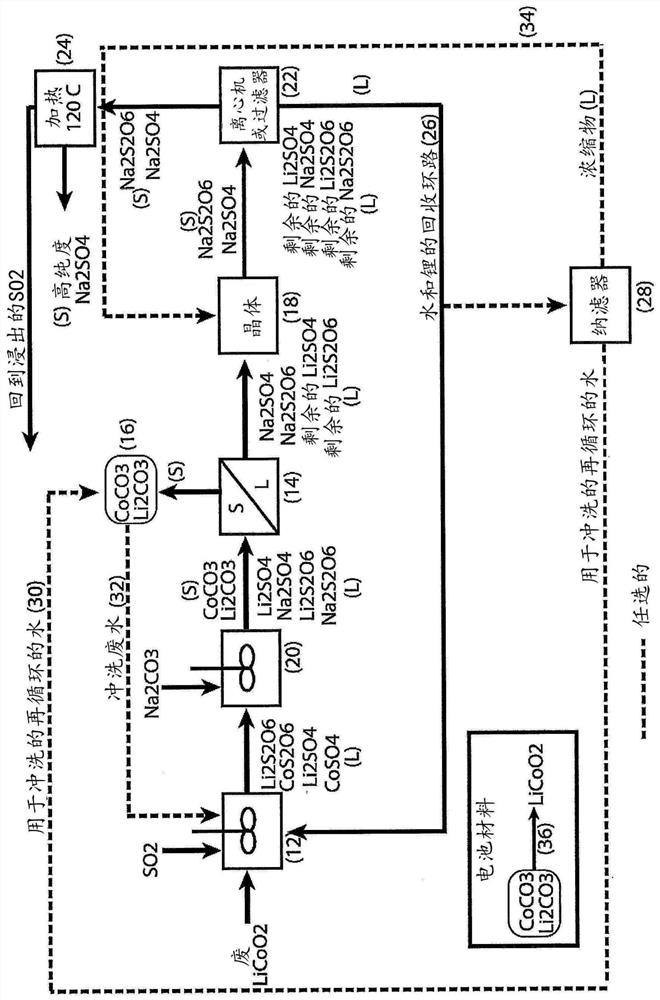
[0036] For the first embodiment, refer to Figure 1-3 .
[0037] A liquor containing cobalt sulfate and cobalt dithionate is treated with sodium carbonate to form cobalt carbonate solids and a liquor containing sodium sulfate and sodium hydrosulfate.
[0038] Lithium sulfate and lithium dithionate, if present, will partially precipitate as lithium carbonate solids along with cobalt carbonate solids.
[0039] Cobalt carbonate-containing solids and lithium carbonate solids, if present, are removed from the carbonate-treated liquor by filtration or centrifugation.
[0040] The cobalt carbonate-containing solid and lithium carbonate solid (if present) are washed to remove soluble impurities and produce a clean material for reuse (eg, cathode material for lithium ion batteries).
[0041] The filtrate or centrate containing sodium sulfate and sodium hydrosulfate and the remainder of lithium sulfate and lithium hydrosulfate (if present) is treated with a crystallizer to crystallize ...
Embodiment approach 2
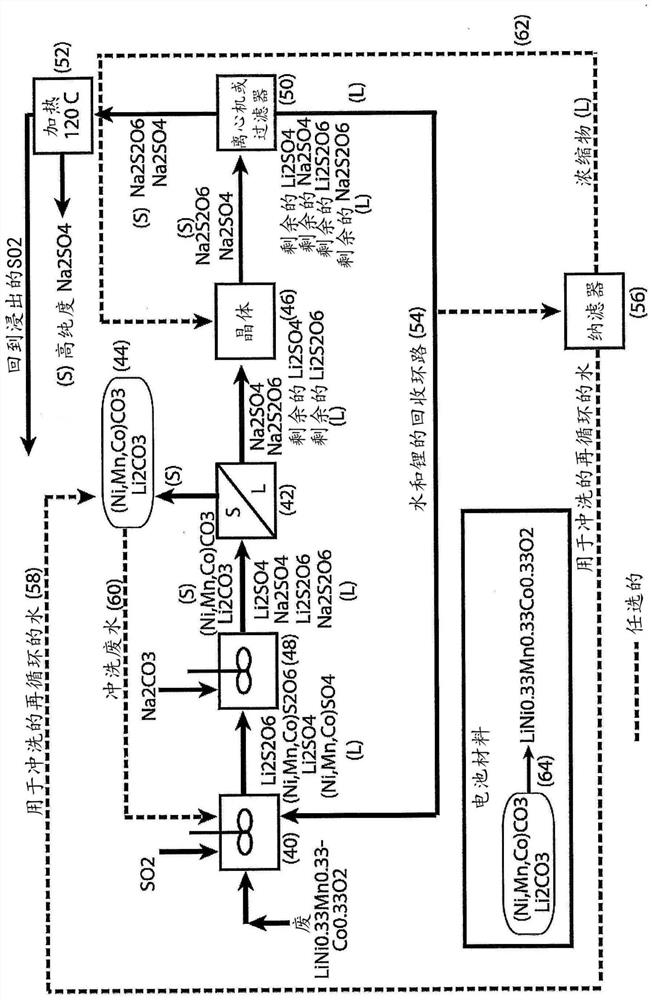
[0048] For the second embodiment, refer to Figure 4-6 .
[0049] Treating a liquor containing cobalt sulfate and cobalt hydrosulfate with sodium hydroxide to form cobalt hydroxide solids and a liquor containing sodium sulfate and sodium hydrosulfate;
[0050] removing solids containing cobalt hydroxide from the hydroxide-treated liquor by filtration or centrifugation;
[0051] washing cobalt hydroxide-containing solids to remove soluble impurities and produce clean materials for reuse (eg, cathode materials for lithium-ion batteries);
[0052] Adding sodium carbonate to the remaining solution precipitates some of the lithium (if present) as lithium carbonate;
[0053] removing lithium carbonate solids from the carbonate-treated liquor by filtration or centrifugation;
[0054] washing the lithium carbonate solids to remove soluble impurities and produce a clean material for reuse (eg, cathode material for lithium ion batteries);
[0055] The filtrate or centrate containing...
Embodiment approach 3
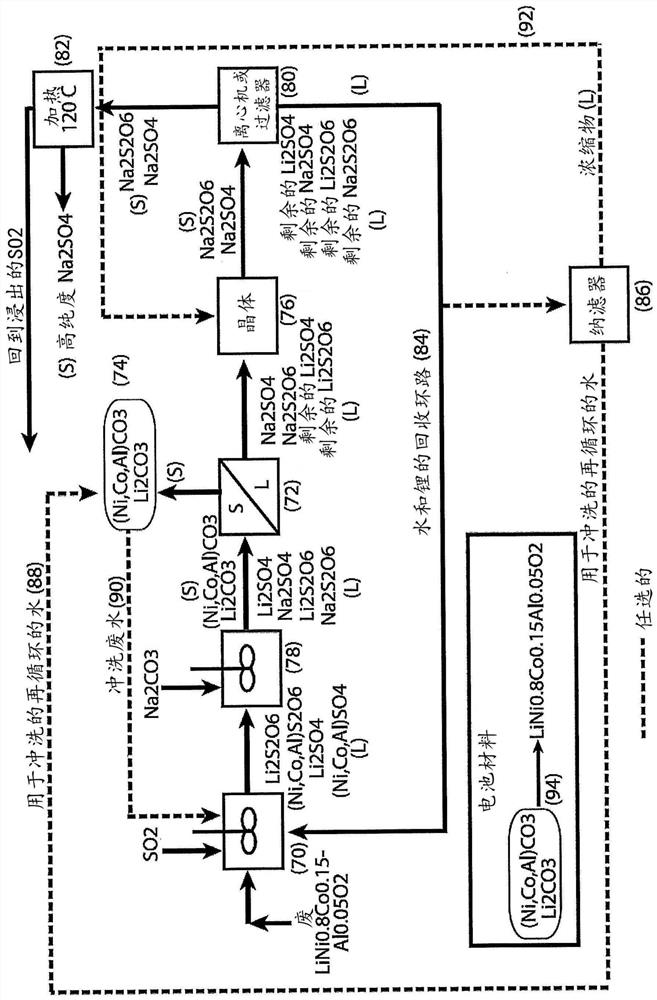
[0060] For the third embodiment, refer to Figure 7-9 .
[0061] treating a liquor containing cobalt sulfate and cobalt dithionate with lithium hydroxide to form cobalt hydroxide solids and a liquor containing lithium sulfate and lithium dithionate;
[0062] Lithium hydroxide can be produced by processing lithium carbonate recovered from previous operations of this flow diagram. Wietelmann et al. ("Lithium and Lithium Compounds", Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co, 2013, p. 24) describe a method for producing lithium hydroxide by reacting lithium carbonate with calcium hydroxide;
[0063] removing solids containing cobalt hydroxide from the hydroxide-treated liquor by filtration or centrifugation;
[0064] washing cobalt hydroxide-containing solids to remove soluble impurities and produce clean materials for reuse (eg, cathode materials for lithium-ion batteries);
[0065] Adding sodium carbonate to the remaining solution precipitates...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com