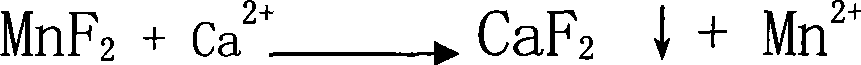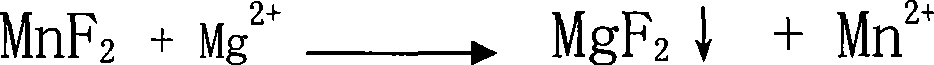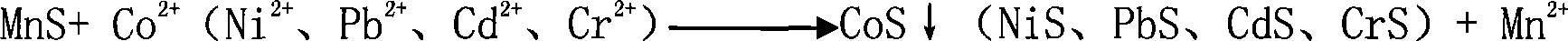Preparation of high purity manganese sulfate
A technology of manganese sulfate and manganese sulfate solution, applied in manganese sulfate and other directions, can solve the problems of low product quality, affecting application, low purity of manganese sulfate products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0011] A method for preparing high-purity manganese sulfate. The solid manganese sulfate containing impurities (calcium and magnesium ion content greater than 100ppm is analyzed by atomic absorption spectroscopy) is prepared into a 100g-300g / l manganese sulfate solution or in manganese ore (calcium , Magnesium ion content is greater than 3000ppm) Manganese sulfate solution (concentration 100g-300g / l) after leaching with sulfuric acid is added with impurity removal agent MnF 2 , In addition to calcium and magnesium impurities, MnF 2 The dosage is 100%-200% of the theoretical dosage of impurity remover required for impurities (calculated according to the reaction equation), maintain the pH of the solution at 3.5-7, and keep it at a temperature of 60℃-100℃ for 0.5 hours-2 hours, and then Add impurity remover MnS or metal manganese powder to remove Co, Ni, Pb, Cd or Cr heavy metal impurities. The amount of MnS or metal manganese powder is 100%-200% of the theoretical amount of impurit...
Embodiment 1
[0014] 200gMnSO 4 (Ca540ppm, Mg360ppm, Co154ppm, Ni76ppm) add water to dissolve to 2000ml, heat and stir to raise to 100℃, add 1g MnF 2 Solid, keep the pH of the solution at 3.5, keep stirring for 1 hour, add 150mg MnS, stir and keep for 0.5 hours, filter, concentrate, crystallize, dehydrate and dry the filtrate to obtain 195g of high-purity manganese sulfate product. The content of manganese sulfate is 99.7. %, in which Ca12ppm, Mg8ppm, Co8ppm, Ni5ppm.
Embodiment 2
[0016] 500gMnSO 4 (Ca540ppm, Mg360ppm, Co154ppm, Ni76ppm) add water to dissolve to 1700ml, heat and stir to raise to 60℃, add 1.5g MnF 2 Solid, add 100mg of metal manganese powder to keep the pH of the solution at 7.0, keep the solution under stirring for 2 hours, filter, concentrate, crystallize, dehydrate and dry the filtrate to obtain 470g of high-purity manganese sulfate product. The analysis of manganese sulfate content is 99.5%, of which Ca5ppm , Mg10ppm, Co5ppm, Ni5ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com