Method for reducing contents of calcium ions, magnesium ions, potassium ions and sodium ions in manganese sulfate
A technology of calcium and sodium ions in manganese sulfate, applied in manganese sulfate and other directions, can solve the problems of difficult control of process conditions, long impurity removal process, unsatisfactory impurity removal effect, etc., and achieves the effect of excellent impurity removal effect and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
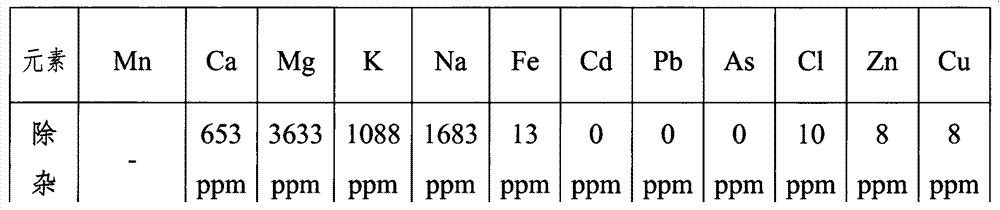
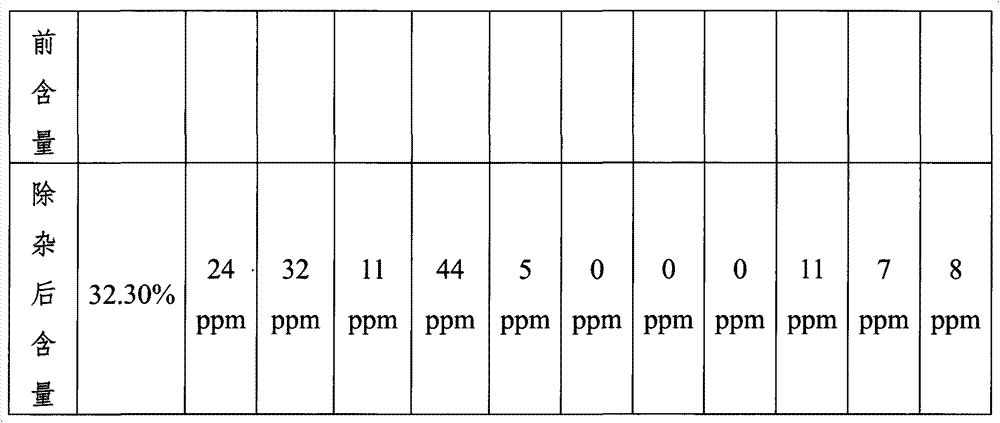
[0029] Get 500g of industrial manganese sulfate solid and dissolve it in water and add water to 1500mL. The concentration of calcium ions in the manganese sulfate solution measured by atomic absorption is 653ppm, the concentration of magnesium ions is 3633ppm, the concentration of sodium ions is 1683ppm, and the concentration of potassium ions is 1088ppm; the solution is heated to 65°C, adjust the pH to 3.5 with 10mol / L sulfuric acid solution; add 27.8g FeF 3 Solid, stir for 1 hour, and filter the precipitate after standing for 6 hours; adjust the pH to 2.0 with 10mol / L sulfuric acid solution, raise the temperature to 95°C, add 10g of jarosite crystals, stir for 2 hours, and filter the crystals after standing at constant temperature for 12 hours ; add 93.8g MnCO 3 The pH of the filtered solution was adjusted to 4.3, and filtered after standing for 24 hours; the filtered solution was concentrated under normal pressure, crystallized, dehydrated, and dried to obtain solid mangane...
Embodiment 2
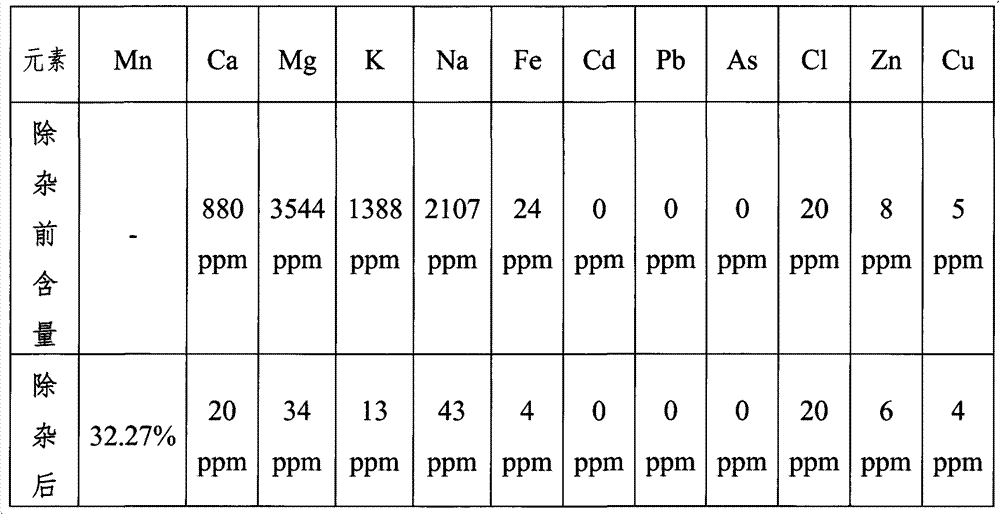
[0033] Take 5kg of industrial manganese sulfate solid and dissolve it in 50L of water. The calcium ion concentration in the manganese sulfate solution measured by atomic absorption is 880ppm, the magnesium ion concentration is 3544ppm, the sodium ion concentration is 2107ppm, and the potassium ion concentration is 1388ppm; the solution is heated to 60°C , adjust the pH to 4.2 with 18.4mol / L sulfuric acid solution; add 0.8kg FeF 3 Solid, stirred for 2 hours, and filtered the precipitate after standing for 6 hours; adjusted the pH to 2.3 with 18.4mol / L sulfuric acid solution, raised the temperature to 95°C, added 200g of jarosite solid, stirred for 3 hours, and kept the crystal at constant temperature for 24 hours Filtration; add 850g Mn(OH) 2 The pH of the filtered solution was adjusted to 4.2, and filtered after standing for 24 hours; the filtered solution was concentrated under normal pressure, crystallized, dehydrated, and dried to obtain solid manganese sulfate. The calciu...
Embodiment 3
[0037] Take 30m 3 Manganese ore sulfuric acid leaching solution, the concentration of manganese sulfate is 104g / L, the concentration of calcium ion in the manganese sulfate solution measured by atomic absorption is 1243ppm, the concentration of magnesium ion is 3672ppm, the concentration of sodium ion is 2202ppm, and the concentration of potassium ion is 1046ppm; Raise the temperature to 60°C, adjust the pH to 3.2 with 18.4mol / L sulfuric acid solution; add 411kg FeF 3 Solid, stirred for 4 hours, filtered the precipitate after standing for 12 hours; adjusted the pH to 1.4 with 18.4mol / L sulfuric acid solution, raised the temperature to 80°C, stirred for 8 hours, and filtered the crystal after standing at constant temperature for 24 hours; added 6.8kgMnCO 3The pH of the filtered solution was adjusted to 4.6, and filtered after standing for 24 hours; the filtered solution was concentrated under normal pressure, crystallized, dehydrated, and dried to obtain solid manganese sulfate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com