Method for high-purity manganese sulfate monohydrate
A technology of manganese sulfate and pure one, applied in the field of preparation of high-purity manganese sulfate monohydrate, can solve the problems of high cost of raw materials and many metal impurities, and achieve the effect of less impurities and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
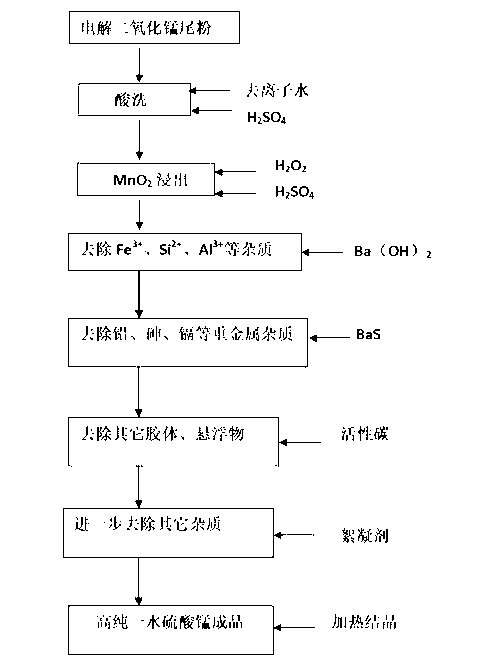
[0025] A. Mix 1,000 grams of electrolytic manganese dioxide tail powder and 2 L of deionized water evenly, add 600 ml of concentrated sulfuric acid to stir and react, and separate solid and liquid; this step removes most of the impurities such as sulfates, carbonates, and sulfides.
[0026] B. Add 1800ml of 30% hydrogen peroxide and an appropriate amount of sulfuric acid to the solid isolate obtained in step A, keep the pH=3, and stir and react at normal temperature for 2 hours.
[0027] C. Gradually add barium hydroxide solution to the solution in step B, and keep stirring until PH = 5.4, then the solid-liquid separation.
[0028] D. According to the Pb content measured in the solution obtained by the solid-liquid separation in step C, add barium sulfide solution with a molar ratio of 2 times, stir evenly, heat to 60-80°C, and separate the solid and liquid.
[0029]
[0030] E. Add the solution obtained from solid-liquid separation in step D into coconut shell activated carb...
Embodiment approach 2
[0035] The difference from Embodiment 1 is that in step B, the pH is kept at 3.5, and the reaction is stirred at room temperature for 4 hours.
Embodiment approach 3
[0037] The difference from Embodiments 1 and 2 is that in step B, the pH is kept at 3, and the reaction is stirred at room temperature for 3 hours.
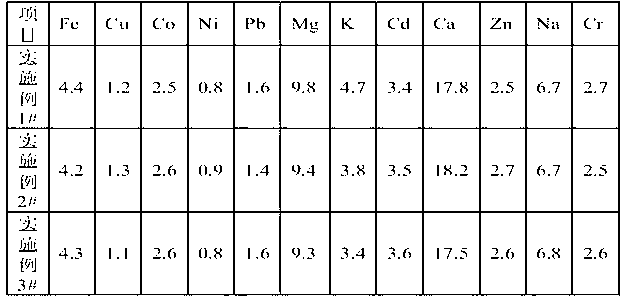
[0038] The various impurity contents that measure after the dried manganese sulfate monohydrate of above each embodiment gained are as following table:
[0039]
[0040] Note: The unit of the data in the table is ppm
[0041] It can be seen from the above table that the impurity content of the high-purity manganese sulfate products obtained in each embodiment of the present invention is relatively low, which can fully meet the needs of lithium battery positive electrode materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com