Method for preparing high-purity manganese sulfate with industrial manganese sulfate as raw material
A technology of industrial sulfuric acid and manganese sulfate, which is applied in the direction of manganese sulfate, etc., can solve the problems that the purity of manganese sulfate is very high and cannot meet the requirements for the use of lithium-ion battery materials, and achieve low cost, good impurity removal effect, and low impurity removal requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
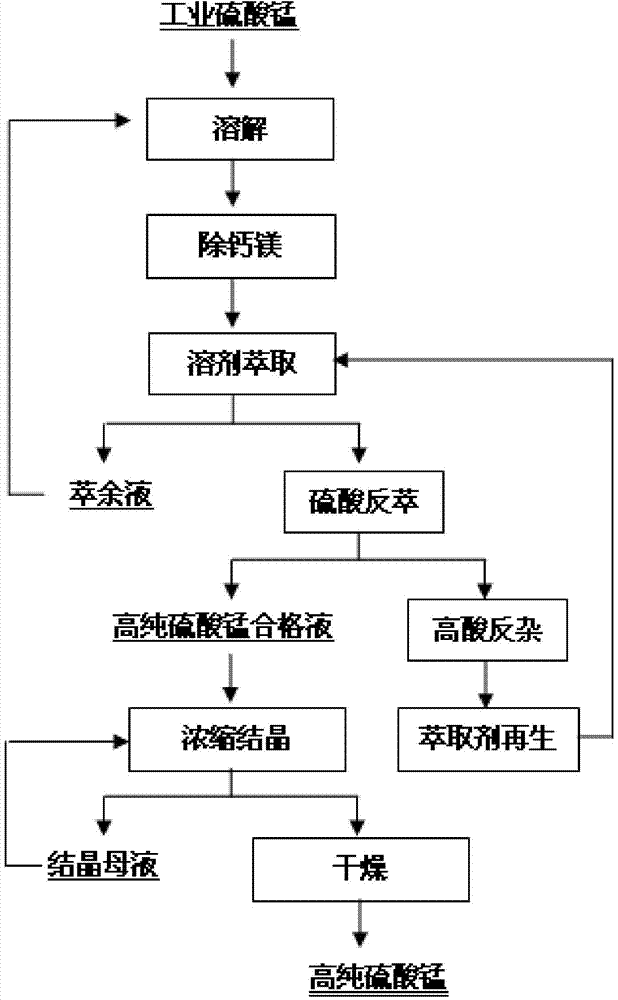
[0022] Take 3000Kg of industrial manganese sulfate, add water and stir to make a fixed solution of 10m 3 , the key components in the solution were measured as shown in Table 1, wherein the manganese concentration was 96.52g / L, and the pH was between 3-6; the manganese sulfate solution was heated to 85°C, sodium fluoride was added, and after 1 hour of reaction, the The calcium ion concentration in the filtered solution is 0.085g / L, and the mass ratio of manganese to calcium is not less than 1000 times to obtain the liquid to be extracted; using 5-stage extraction, the flow ratio of the organic and the liquid to be extracted is controlled to be 3:1, and the organic Compared with the liquid to be extracted, it is 1:1, and the extraction agent is a phosphate-based extractant; the pH of the raffinate is 2.5, and it is returned to dissolve industrial-grade manganese sulfate; 2.5N sulfuric acid is used to pass the 5-stage back-extraction organic phase to control the back-extraction T...
Embodiment 2
[0030] Take 1500Kg of industrial manganese sulfate, dissolve the raffinate and set the solution to 10m 3 , the key components in the measured solution are shown in Table 4, wherein the manganese concentration is 87.23g / L; the manganese sulfate solution is heated to 80°C, potassium fluoride is added, and after 2 hours of reaction, the calcium ion in the filtered solution is measured The concentration is 0.078g / L, the mass ratio of manganese to calcium is not less than 1000 times, and the liquid to be extracted is obtained; 6-stage extraction is used to control the flow ratio of the organic to the liquid to be extracted to 2:1, and the ratio of the organic to the liquid to be extracted is 2: 1. Phosphate ester extractant was used as the extractant; the raffinate had a pH of 2.0, and returned to dissolve industrial-grade manganese sulfate; 3.0N sulfuric acid was used to pass through the 5-stage stripping organic phase, and the outlet pH of the stripping solution was controlled to ...
Embodiment 3
[0038] Take industrial manganese sulfate and add water to stir so that the manganese concentration is controlled at 80g / L, and the pH is between 3-6; the manganese sulfate solution is heated to 90°C, and ammonium fluoride is added, and after 1 hour of reaction, the mass ratio of manganese and calcium in the solution is not Less than 1000 times, get the liquid to be extracted; use 6-stage extraction, control the flow ratio of organic and liquid to be extracted to 5:1, the ratio of organic to liquid to be extracted is 1:2, and the extraction agent uses phosphate ester extractant; raffinate Liquid pH is 3.0, return to dissolve industrial-grade manganese sulfate; use 4.0N sulfuric acid to pass through 6-stage back-extraction organic phase, control stripping liquid outlet pH to be 3.0, obtain manganese sulfate qualified liquid, after testing, manganese and calcium, magnesium, iron The mass ratio multiples of zinc, sodium, and potassium are not less than 10,000, 10,000, 30,000, 20,00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com