Method for preparing high-purity manganese sulfate from manganese-containing waste liquid
A technology of manganese sulfate and waste liquid, applied in the direction of manganese sulfate, etc., can solve the problems of low utilization value of manganese hydroxide, difficult solid waste treatment, environmental pollution, etc., to reduce the amount of waste residue, reduce the difficulty of treatment, and increase the separation coefficient Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] experiment procedure
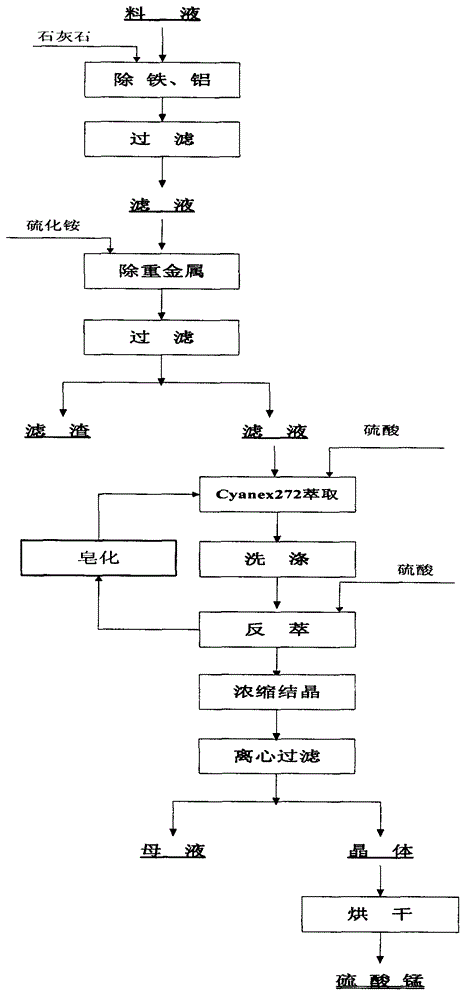
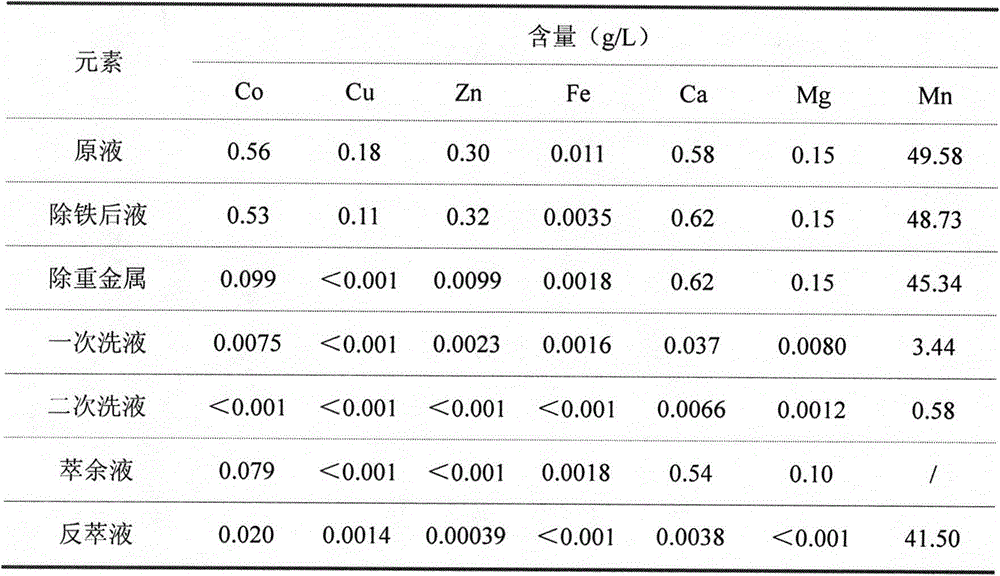
[0029] 1) Adjust the pH of the feed solution to 4.5 with limestone, react for 2 hours, and filter;
[0030] 2) When the temperature is 50°C, add ammonium sulfide to the filtrate to remove heavy metals, add sulfuric acid appropriately, control the pH at about 4.5, react for 1 hour, and filter;
[0031] 3) The pH of the filtrate is adjusted back to 3.5 as the extraction feed liquid;
[0032] 4) Mix 400mL Cyanex272 organically with 1600mL kerosene, the effective organic content is 25% (volume ratio);
[0033] 5) Weigh 25.2g of NaOH solid and dissolve it into 58.7g of distilled water to form caustic soda with a concentration of 30%;
[0034] 6) Add caustic soda to the mixed organic, and carry out homogeneous saponification to the organic, and the saponification rate is 50%;
[0035] 7) Organic 280mL, water phase 100mL, organic extraction of water phase twice, after reaching saturation, use 50mL of dilute acid with pH=4.0 to wash the organic twice, ...
example 2
[0043] experiment procedure
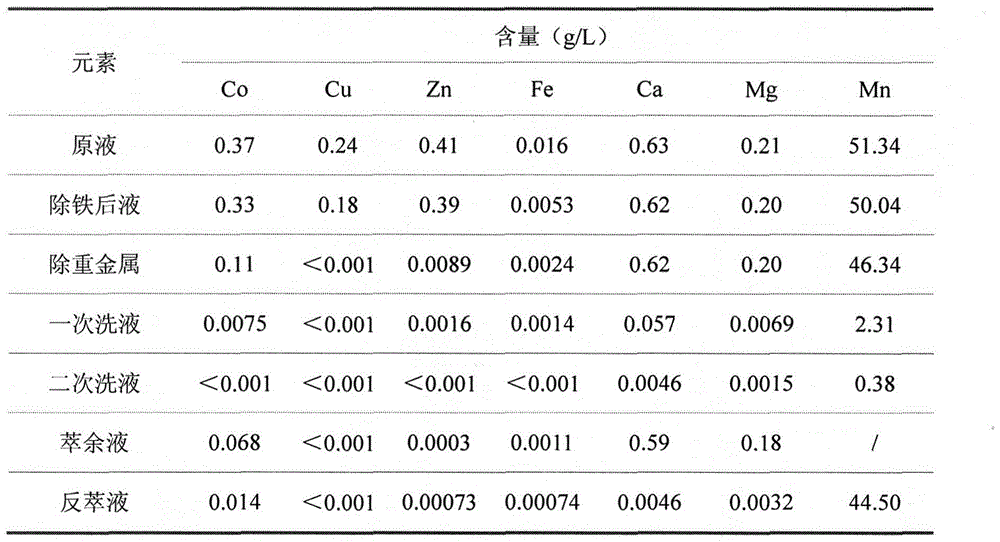
[0044] 1) Adjust the pH of the feed liquid to 3.0 with limestone, react for 2 hours, and filter;
[0045] 2) When the temperature is 50°C, add ammonium sulfide to the filtrate to remove heavy metals, add sulfuric acid appropriately, control the pH at about 4.0, react for 1 hour, and filter;
[0046] 3) The pH of the filtrate is adjusted back to 3.0 as the extraction feed liquid;
[0047] 4) Mix 400mL Cyanex272 organically with 2000mL kerosene, the effective organic content is 20% (volume ratio);
[0048] 5) Weigh 25.2g of NaOH solid and dissolve it into 58.7g of distilled water to form caustic soda with a concentration of 30%;
[0049] 6) Add caustic soda to the mixed organic, and carry out homogeneous saponification to the organic, and the saponification rate is 55%;
[0050] 7) Organic 300mL, water phase 100mL, organic extraction of water phase twice, after reaching saturation, use 50mL of dilute acid with pH=4.5 to wash the organic twice, us...
example 3
[0058] experiment procedure
[0059] 1) Adjust the pH of the feed liquid to 5.5 with limestone, react for 2 hours, and filter;
[0060] 2) When the temperature is 50°C, add ammonium sulfide to the filtrate to remove heavy metals, add sulfuric acid appropriately, control the pH at about 6.0, react for 1 hour, and filter;
[0061] 3) The pH of the filtrate is adjusted back to 4.0 as the extraction feed liquid;
[0062] 4) Mix 400mL Cyanex272 organically with 1600mL kerosene, the effective organic content is 25% (volume ratio);
[0063] 5) Weigh 25.2g of NaOH solid and dissolve it into 58.7g of distilled water to form caustic soda with a concentration of 30%;
[0064]6) Add caustic soda to the mixed organic, and carry out homogeneous saponification to the organic, and the saponification rate is 50%;
[0065] 7) Organic 280mL, water phase 100mL, organic extraction of water phase 2 times, after reaching saturation, use 50mL of dilute acid with pH=4.5 to wash the organic twice, u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com