Method for preparation of high purity manganese sulfate and zinc sulfate from waste zinc-manganese batteries
A zinc-manganese battery and manganese sulfate technology are applied in the comprehensive utilization of waste resources and the recycling and utilization of valuable metals in waste zinc-manganese batteries, which can solve the problems of failing to meet the application requirements in the application field and low added value of products, and achieve comprehensive utilization. The effect of strong capacity, high recovery value and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
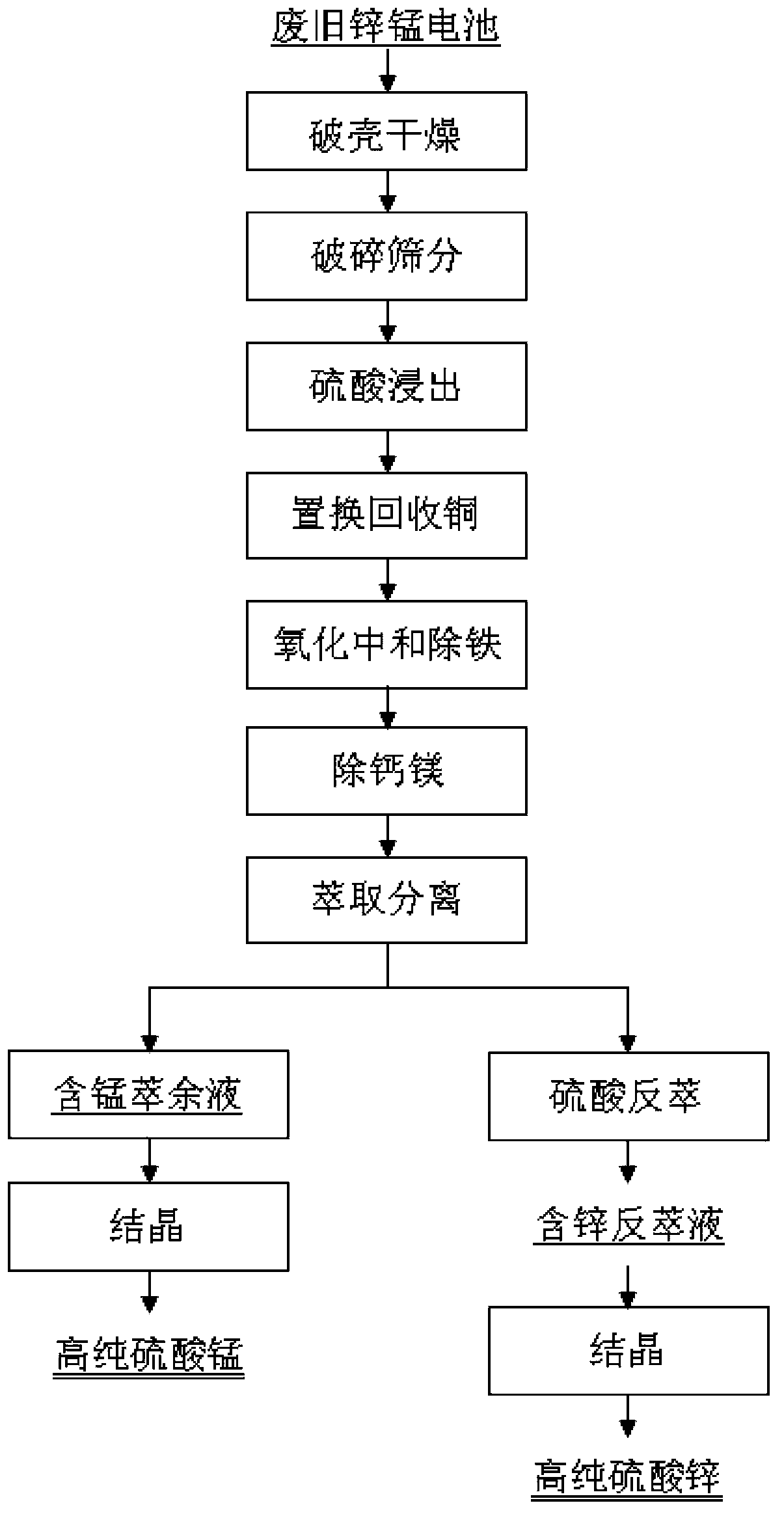
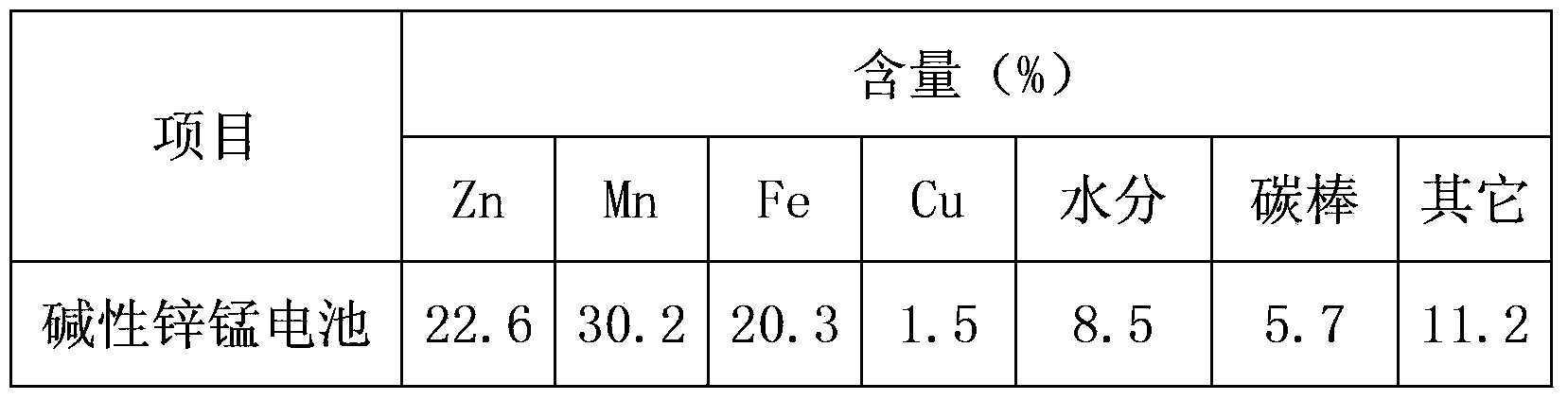
[0029] Dry the waste alkaline zinc-manganese batteries at 120°C, crush them into powder, and pass through 20 meshes; the main metal analysis of the underscreen is shown in Table 1 below.
[0030] Table 1 Alkaline battery component content
[0031] components
Zn
mn
Fe
Cu
Content (%)
22.6
30.2
20.3
1.5
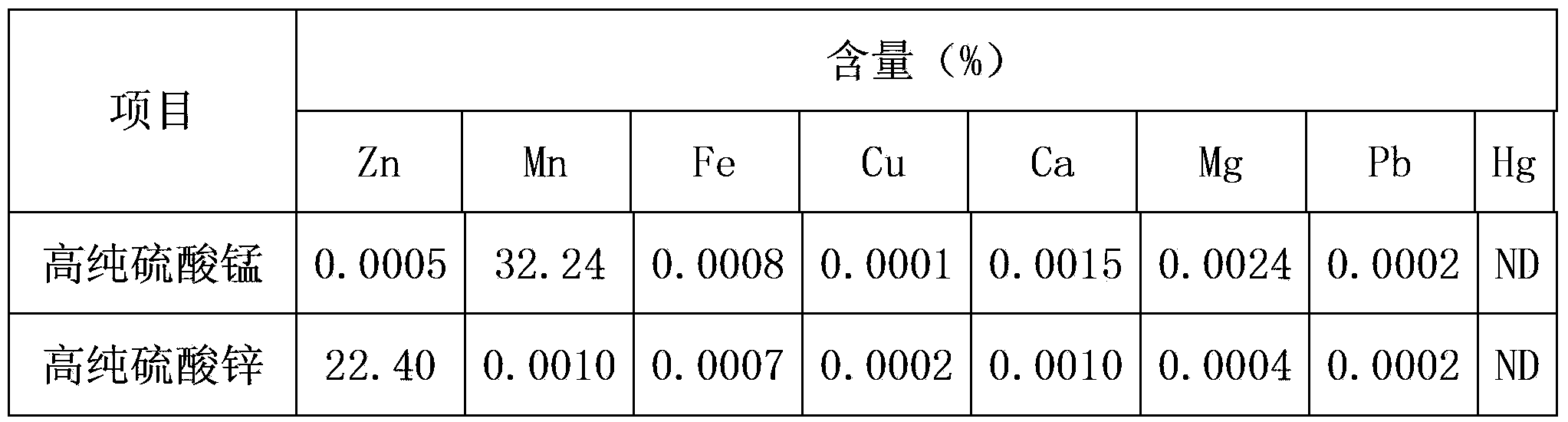
[0032] Take the undersieve, use 2mol / L sulfuric acid to carry out acid leaching, the reaction temperature is 60 degrees, and when the pH of the reaction end reaches 2.0, filter to obtain the acid leaching filtrate. According to the copper content in the solution, add reduced iron powder according to 1.2 times the theoretical amount to replace the copper in the pickling solution, and when the copper concentration reaches the requirement, filter to obtain the copper-removing filtrate and by-product copper powder. Introduce oxygen into the copper removal filtrate to oxidize ferrous iron in the solution to ferric iron, then rai...
Embodiment 2
[0038] Dry the waste acidic zinc-manganese battery at 100°C, crush it into powder, and pass through a 50-mesh sieve; take the undersieve, use 2mol / L sulfuric acid for acid leaching, the reaction temperature is 50 degrees, and when the pH of the reaction end point reaches 1.5, filter to obtain Acid leaching filtrate. According to the copper content in the solution, add reduced iron powder according to 1.3 times the theoretical amount to replace the copper in the pickling solution. When the copper concentration reaches the requirement, filter the copper removal solution and by-product copper powder. Introduce oxygen into the copper removal solution to oxidize ferrous iron in the solution to ferric iron, then raise the temperature to 90°C, slowly add manganese carbonate, adjust the pH to 4.0, and maintain the reaction for 6 hours, then filter to obtain the iron-removed solution . An appropriate amount of sodium fluoride is added to the solution after iron removal according to th...
Embodiment 3
[0044]Randomly take a pile of waste zinc-manganese batteries, dry them, crush them into powder, and pass through an 80-mesh sieve; take the undersieve, use 1.5mol / L sulfuric acid for acid leaching, the reaction temperature is 70 degrees, and when the pH of the reaction end point reaches 2.0, filter Acid leaching filtrate was obtained. According to the copper content in the solution, add reduced iron powder according to 1.2 times the theoretical amount to replace the copper in the pickling solution. When the copper concentration reaches the requirement, filter the copper removal solution and by-product copper powder. Introduce oxygen into the copper removal solution to oxidize ferrous iron in the solution to ferric iron, then raise the temperature to 85°C, slowly add manganese carbonate, adjust the pH to 4.5, and maintain the reaction for 12 hours, then filter to obtain the iron-removed solution . An appropriate amount of sodium fluoride is added to the solution after iron rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com