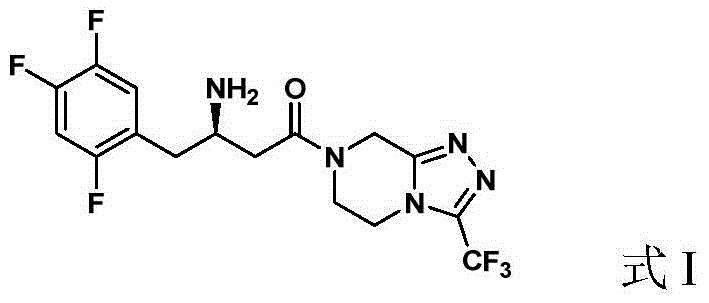
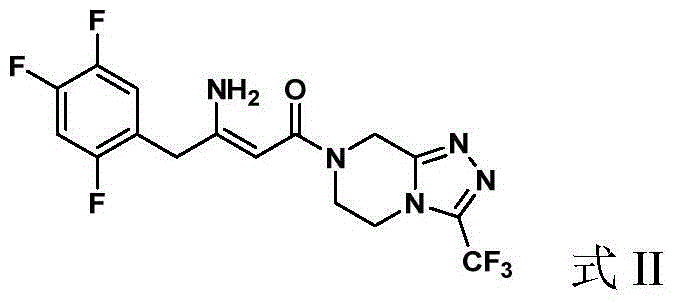
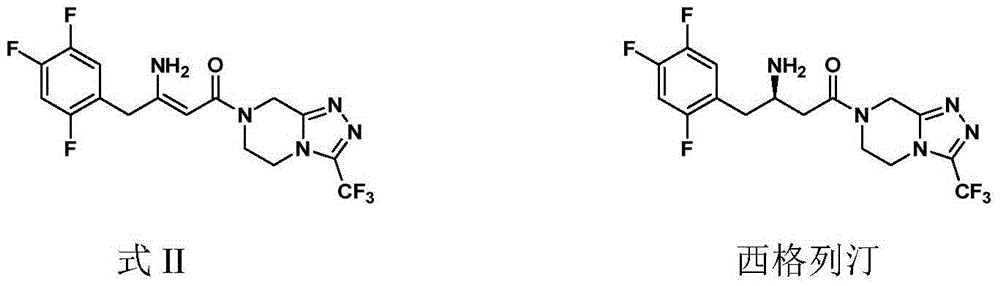Preparation of Sitagliptin
A technology selected from and compound, applied in the direction of organic chemistry, can solve the problems of high price, long hydrogenation reaction time, unsuitable for scale-up production, etc., and achieve the effect of short reaction time and high ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthesis of embodiment 1 formula IV compound
[0046]
[0047] Add 2,4,5-trifluorophenylacetic acid (5.5kg, 28.93mol), McFarlandic acid (4.62kg, 31.8mol), DMAP (0.283kg, 2.3mol) and acetonitrile (16.5L ). DIEA (11L, 62.2mol) was added dropwise to the above suspension, and then t-BuCOCl (3.92L, 31.8mol) was slowly added dropwise. After the addition, the temperature was kept at 45-50°C for 2-3 hours. After the reaction is finished, there is no need to deal with and continue to the next step of synthesis.
Embodiment 2
[0048] The synthesis of embodiment 2 formula III compound
[0049]
[0050] Add 3-(trifluoromethyl)-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyrazine to the previous reaction system (Example 1) Hydrochloride (6.62kg, 29.04mol), then slowly add CF 3COOH (0.66L, 8.69mol), after the dropwise addition, set the reaction temperature to 50-55°C for about 6 hours. After the reaction, no treatment is required to continue the next step of synthesis.
Embodiment 3
[0052] (2Z)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7( Synthesis of 8H)-yl]-1-(2,4,5-trifluorophenyl)but-2-en-2-amine (Formula II)
[0053]
[0054] Add ammonium acetate (2kg, 26mol), ammonia water (4L) and methanol (59.4L) respectively in the 200L reaction kettle, this solution is warmed up to 45 ℃, takes 10% volume of previous step reaction solution (Example 2) dropwise added to the above solution. After the dropwise addition, the reaction was maintained at 45° C. for 2.5 hours. Continue to add the remaining 90% of the reaction solution dropwise. After the dropwise addition was completed, the reaction was continued for 3 hours, and then methanol (26.4 L) was added dropwise. After the dropwise addition, the temperature of the system was slowly lowered to 0-5°C. And continue to stir at this temperature for 1 hour, then shake off and filter. Add the filter cake into a 100L reaction kettle, add purified water, stir and make a slurry for 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com