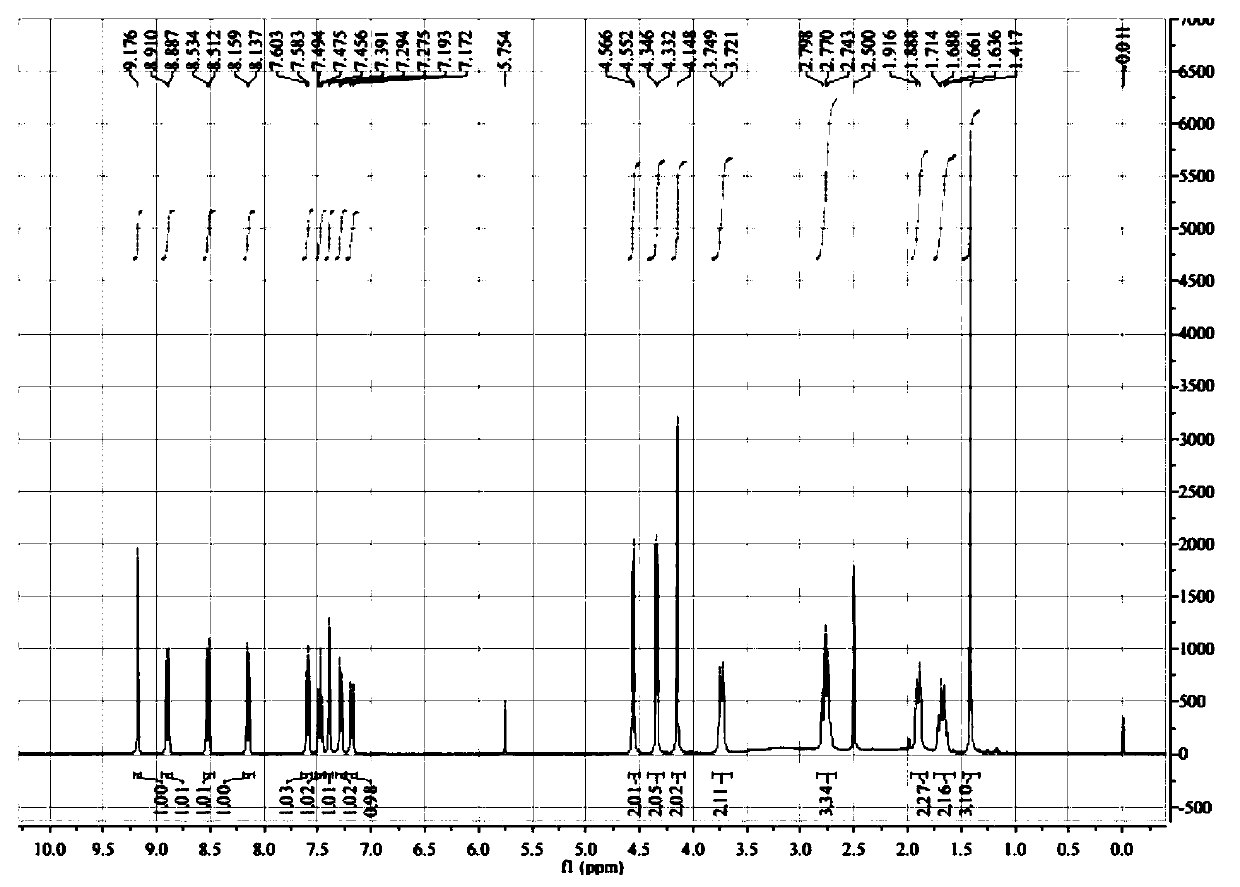
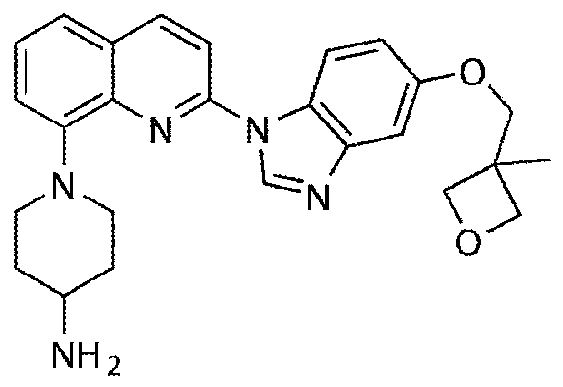
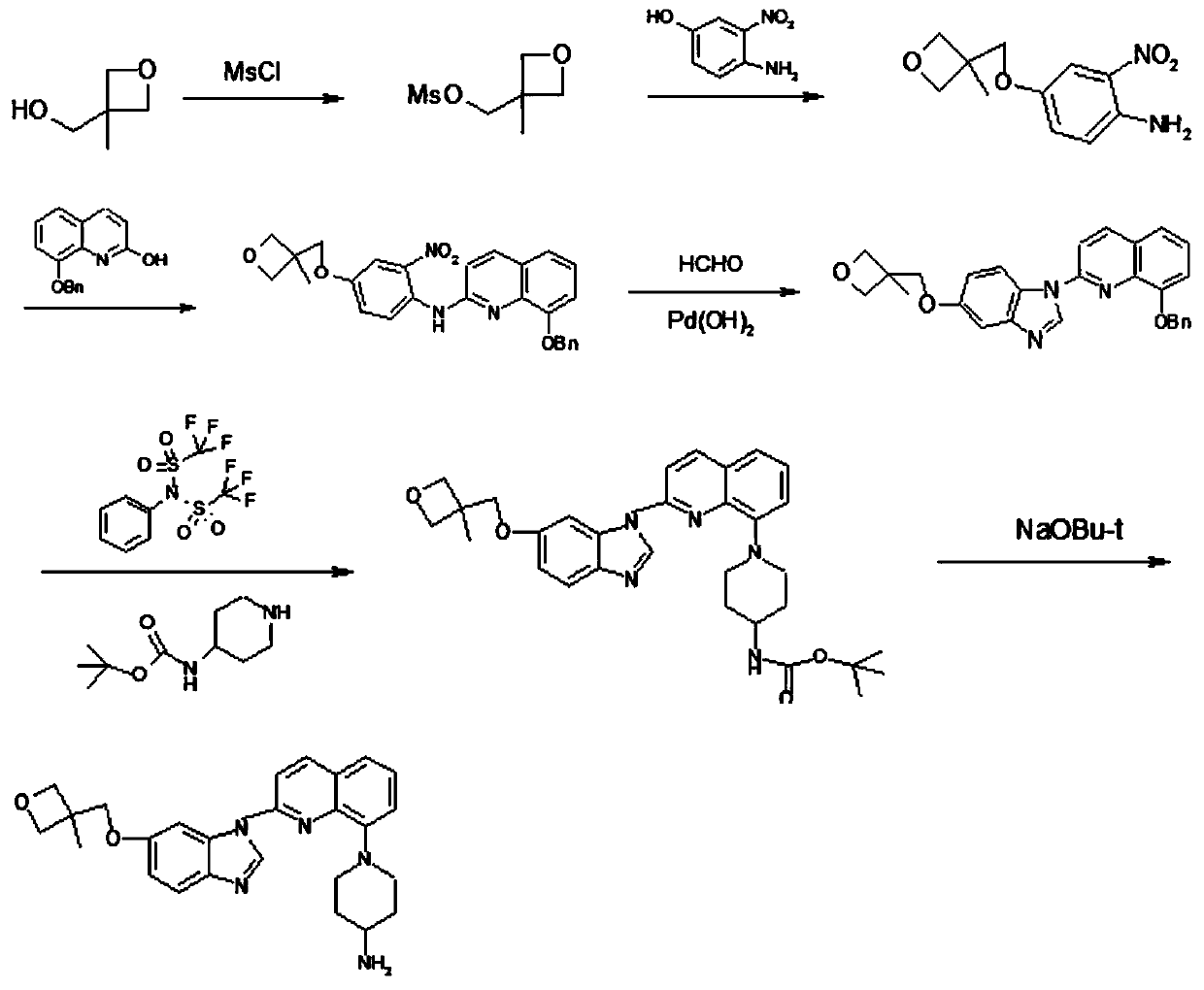
A kind of synthesis method of medicine for treating leukemia
A synthesis method and compound technology, applied in the field of preparation of pharmaceutical compounds, can solve problems such as unsuitable for industrial production, environmental pollution, explosive and toxic, etc., and achieve the effects of being conducive to industrial production, safe operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0088] Preparation of 4(3-methyl-oxetan-3-ylmethoxy)-2-nitro-phenylamine (A1)
[0089] Add 28g of compound 3-methyl-3-oxetanemethanol, THF140ml, and metal sodium 6.32g into a 250ml three-necked flask, heat to 66°C for 4h under stirring, cool to 55°C, add 4-amino- 40.4g of 3-nitrochlorobenzene, heated under stirring, controlled temperature at 50-60°C for 24 hours, added 180ml of ethyl acetate into the reaction bottle, stirred at 35-40°C for 0.5h, then filtered, and washed the filter cake with 180mlx2 , the filtrate was washed with 500ml of 0.5N sodium hydroxide and a saturated solution of sodium chloride respectively, the organic phase was dried with 30g of anhydrous magnesium sulfate for 0.5h, the desiccant was filtered off, the filtrate was concentrated under reduced pressure, and 140ml of isopropanol was added to the concentrated residue. Stir at 15-20°C for 10h, filter the precipitated solid, and blow dry at 40°C to obtain 45.28g of a reddish-orange solid, with a yield of 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| stone rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com