Synthesis method of (R)-5'-methoxyl laudanosine
A technology of methoxylabadenin and synthesis method, applied in the directions of organic chemistry, organic chemistry, etc., can solve the problems of genotoxic impurity residue, low catalyst selectivity, etc., and achieves easy large-scale production, and the technological operation process is not harsh. , the effect of high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
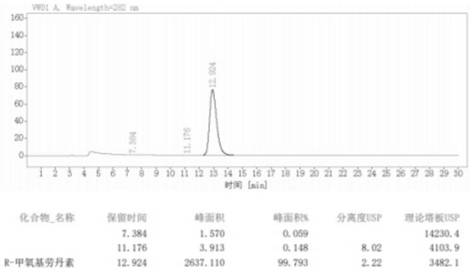
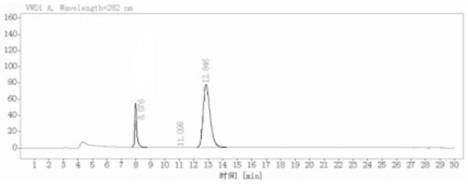
Image
Examples
Embodiment 1
[0050] A kind of synthetic method of (R)-5'-methoxylaudansu in the embodiment of the present invention: the reagents of N-formylation reaction are formic acid and zinc powder, and the product purification solvent of N-formylation reaction is ethanol ; The asymmetric hydrogenation reduction reaction adopted methanol-dichloromethane mixed solvent; the formyl reduction adopted lithium aluminum hydride as a reducing agent; the total yield of the reaction route was 45.86%, and the obtained (R)-5'-methoxy The optical purity of baudansu is 99.79%; the specific synthesis method is as follows:
[0051] (1) Synthesis of (Z)-N-formyl-6,7-dimethoxy-1-(3,4,5-trimethoxybenzylidene)-3,4-dihydroisoquinoline :
[0052] Weigh 37.2g (0.1mol) of 6,7-dimethoxy-1-(3,4,5-trimethoxybenzyl)-3,4-dihydroisoquinoline into a three-necked flask, add 151g of formic acid (3.28mol), after stirring and dissolving, add 0.66g (0.01mol) of zinc powder, use a 500W tungsten lamp to irradiate and heat, stir and re...
Embodiment 2
[0059]A kind of synthetic method of (R)-5'-methoxylaudansu in the embodiment of the present invention: the reagent of N-formylation reaction is formic acetic anhydride, the product purification solvent of N-formylation reaction is methanol; Symmetric hydrogenation reduction reaction adopted methanol-dichloromethane mixed solvent; formyl reduction adopted rhodium chloride trihydrate-diphenylsilane as reducing agent; reaction route total yield was 59.78%, obtained (R)- The optical purity of 5'-methoxylaudansin is 99.80%; the specific synthesis method is as follows:
[0060] (1) Synthesis of (Z)-N-formyl-6,7-dimethoxy-1-(3,4,5-trimethoxybenzylidene)-3,4-dihydroisoquinoline :
[0061] Weigh 37.2g (0.1mol) of 6,7-dimethoxy-1-(3,4,5-trimethoxybenzyl)-3,4-dihydroisoquinoline into a three-necked flask, add formic acetic anhydride 22g (0.25mol), tetrahydrofuran 100mL, triethylamine 50.1g (0.5mol), use 500W tungsten lamp to irradiate and heat, stir and react at 70-75°C for 2.0-2.5 hou...
Embodiment 3
[0067] A kind of synthetic method of (R)-5'-methoxylaudansu in the embodiment of the present invention: the reagent of N-formylation reaction is 2,2-dimethylpropionic formic anhydride, N-formylation The product purification solvent of reaction is ethanol; What asymmetric hydrogenation reduction reaction adopts is ethanol-dichloromethane mixed solvent; What formyl reduction adopts is rhodium chloride trihydrate-diphenylsilane as reducing agent; Reaction route total yield is 81.9%, the optical purity of (R)-5'-methoxylaudansin obtained is 99.82%; the specific synthesis method is as follows:
[0068] (1) Synthesis of (Z)-N-formyl-6,7-dimethoxy-1-(3,4,5-trimethoxybenzylidene)-3,4-dihydroisoquinoline :
[0069] Weigh 37.2 g (0.1 mol) of 6,7-dimethoxy-1-(3,4,5-trimethoxybenzyl)-3,4-dihydroisoquinoline into a three-necked flask, add 2, 27.5g (0.21mol) of 2-dimethylpropionic formic anhydride, 150mL of dichloromethane, and 34.6mLg (0.43mol) of pyridine were stirred and reacted at 23-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com