Heterogeneous asymmetric hydrogenation catalyst and application thereof
A hydrogenation catalyst, asymmetric technology, applied in physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, organic chemistry, etc. Poor stability or thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
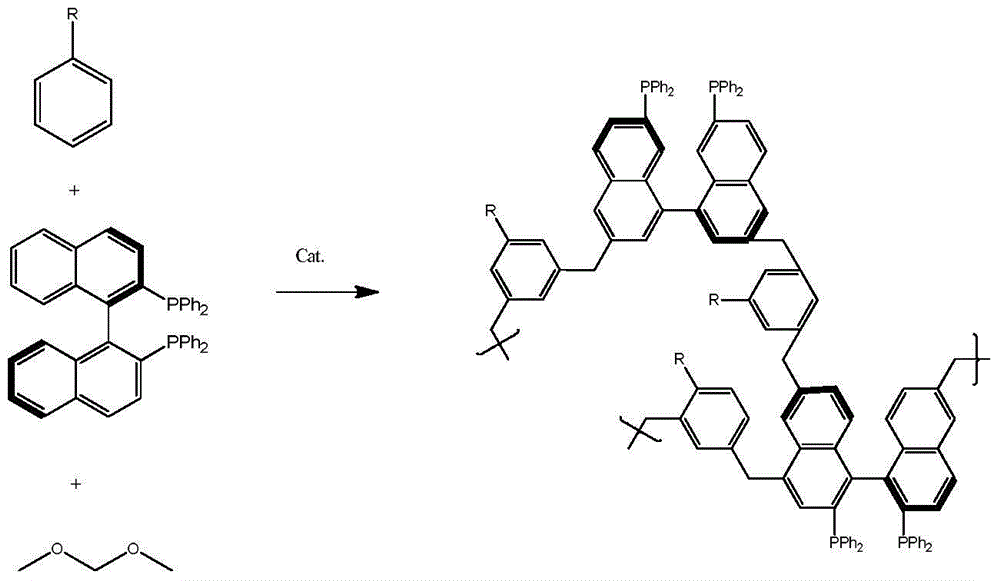
[0016] Taking the comonomer benzene as an example, the preparation method of the catalyst is illustrated. Specific steps are as follows:
[0017] (1) Add 0.249gBINAP and 4.0gAlCl 3 Add about 25ml of dichloromethane in the flask, then add 0.781g of benzene and 2.28g of dimethoxymethane in sequence. After stirring evenly, stand at 80°C for 67h to obtain a polymer.
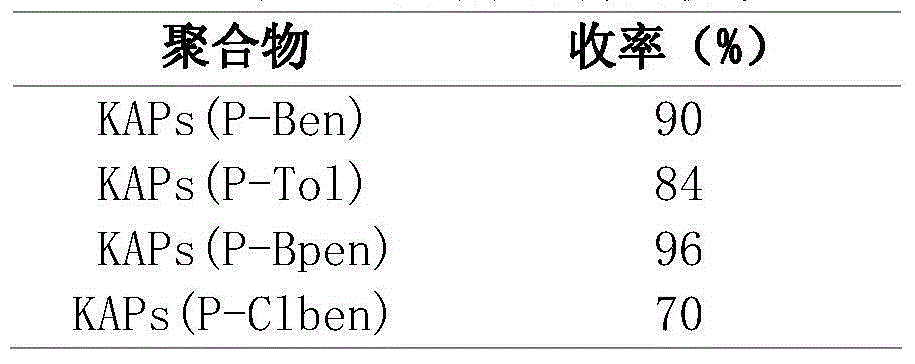
[0018] (2) Wash the polymer obtained in step 1 with methanol for 24 hours. Vacuum-dried at 60°C for 6 hours, and the obtained polymer was denoted as KAPs (P-Ben).
[0019] (3) Take 0.8g of the material obtained in step 2, add 0.0080gRu 2 (C 6 h 6 ) 2 Cl 4 , dichloromethane as solvent, after stirring at room temperature for 24h, vacuum-dried at 60°C for 6h. The prepared catalyst number is Ru / KAPs(BINAP)-1.
Embodiment 2
[0021] Filled with 0.249gBINAP and 2.66gAlCl 3 Add about 15ml of dichloromethane in the flask, then add 0.921g of toluene, 1.522g of dimethoxymethane successively. After stirring evenly, stand at 80°C for 67h to obtain a polymer. After washing with methanol for 24 hours, vacuum-drying at 60°C for 6 hours, the obtained polymer was designated as KAPs(P-Tol).
Embodiment 3
[0023] Filled with 0.250gBINAP and 5.33gAlCl 3 Add about 15ml of dichloromethane in the flask, then add 1.542g of biphenyl, 3.044g of dimethoxymethane successively. After stirring evenly, stand at 80°C for 67h to obtain a polymer. After washing with methanol for 24 hours, vacuum-drying at 60°C for 6 hours, the obtained polymer was designated as KAPs (P-Bpen).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com