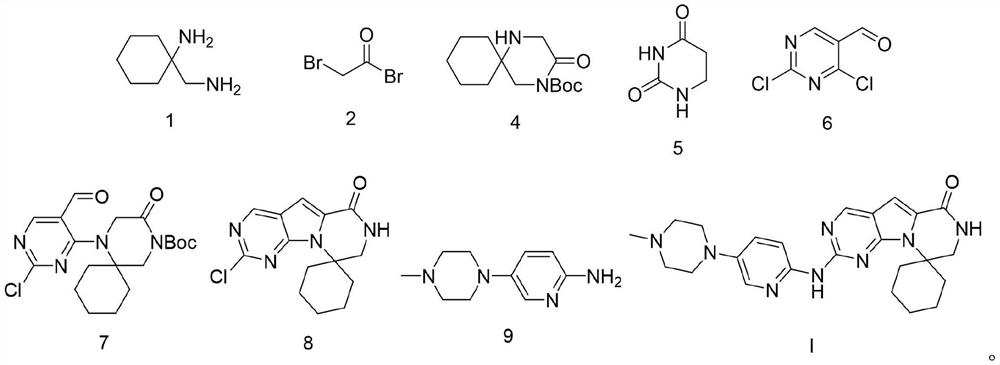
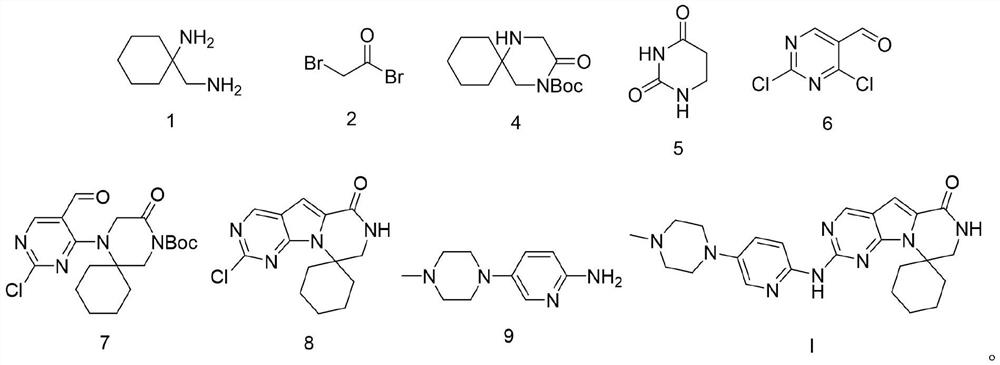
Preparation process of trirasilil compound
A technology for tralaciride and preparation process is applied in the field of preparation of tralaciride compounds, which can solve the problems such as the preparation process of tralacilide compounds needs to be improved, and achieves improvement of total yield and industrial operability, simple reaction operation, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
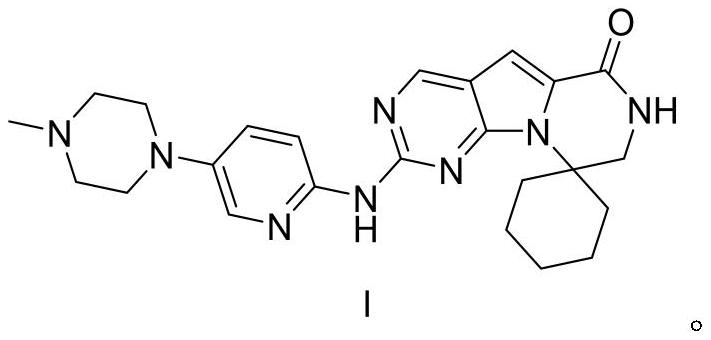
[0072] The preparation of compound Triracilil shown in embodiment 9 formula I
[0073] At room temperature, add the compound shown in formula 8 (29.08g, 0.1mol) to dioxane (300mL), stir, add the compound shown in formula 9 (21.15g, 0.11mol), tBuOK (16.83g, 0.15mol) ), tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 , 4.58g, 0.005mol), 1,1'-binaphthyl-2,2'-bisdiphenylphosphine (BINAP, 6.23g, 0.01mol), after nitrogen replacement for three times, stir and heat up to 100°C for 2h, then The reaction mixture was kept at 20°C to 25°C and stirred for 18h, then ethyl acetate (300ml) was added to the reaction solution, stirred evenly, filtered with diatomaceous earth, the filter cake was washed with ethyl acetate, and the obtained filtrate was collected and decompressed Concentrate, the concentrate that obtains adopts the mixed solvent that the dichloromethane / methanol of volume ratio is 20:1 to carry out silica gel column chromatography purification, collects the filtrate that ob...
Embodiment 12
[0080] Example 12 is a comparative example. In this example, the inventor adjusted the compound shown in formula 6 and triethylamine and the compound shown in formula 4 to slowly raise the temperature to 50°C and continue to stir for 2.5h. In terms of technical effect, this implementation The product yield (63.5%) that example obtains is significantly lower than the product yield (72.7%) when compound shown in formula 6 described in embodiment 5 and triethylamine, formula 4 are slowly warming up to 60 ℃ and continue to stir for 2h. %).
[0081] At room temperature, the compound shown in formula 6 (10g, 0.0565mol), Et 3 N (6.86g, 0.0678mol) was added into THF (80mL), and kept at 20°C to 25°C, slowly added dropwise a mixture containing the compound shown in formula 4 (15.91g, 0.0593mol) and THF (40mL), and The reaction temperature of the mixed system is kept at 20°C to 25°C. After stirring for 0.5 hours, slowly raise the temperature to 50°C and continue to stir for 2.5 hours. T...
Embodiment 13
[0083] Embodiment 13 is a comparative example. In this embodiment, the inventor adjusted the compound shown in formula 8, the compound shown in formula 9, tBuOK, Pd 2 (dba) 3 , the mol ratio of BINAP is 1:1.4:4:0.015:0.2, and its technical effect, the product yield obtained is significantly lower than the compound shown in formula 8, the compound shown in formula 9, tBuOK, Pd 2 (dba) 3 , the product yield when the molar ratio of BINAP is 1:(1.0~1.25):(1.2~3):(0.02~0.1):(0.05~0.15), and the HPLC purity of the triracilide product obtained obviously reduces, Impurities in the product increase.
[0084] At room temperature, add the compound shown in formula 8 (29.08g, 0.1mol) to dioxane (300mL), stir, add the compound shown in formula 9 (26.92g, 0.14mol), tBuOK (44.88g, 0.4mol ), Pd 2 (dba) 3 (1.37g, 0.0015mol), BINAP (12.45g, 0.02mol), after nitrogen replacement three times, stirred and heated up to 100°C for 2h, then kept the reaction mixture at 20°C~25°C and stirred for 18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com