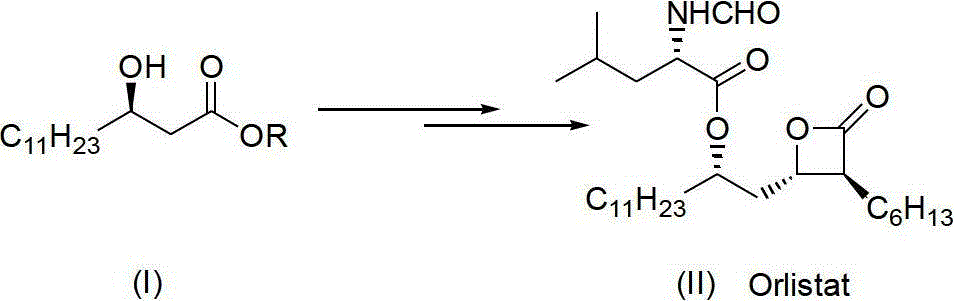
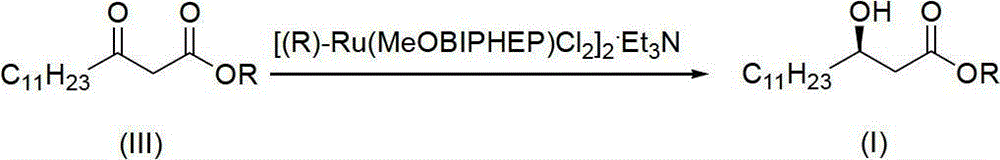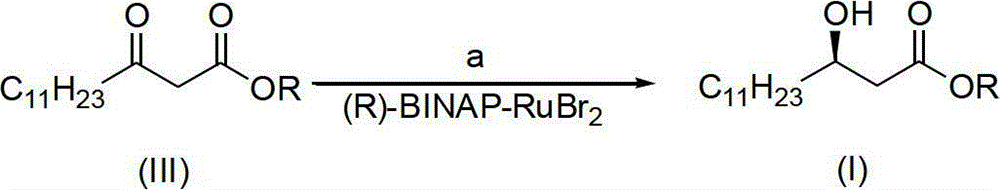Method for synthesizing (R)-beta-hydroxytetradecanoate
A technology of hydroxytetradecanoate and -binap-rubr2, which is applied in the field of synthesizing -β-hydroxytetradecanoate, can solve the problems that patients are unbearable, natural products are difficult to obtain, and production costs are high, and achieves low cost, Reaction conditions are easy to achieve, avoiding the effect of acid-resistant equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of (R)-β-hydroxytetradecanoic acid methyl ester, the compound of formula (I) (R=Me)
[0021] Proceed as follows:
[0022] (1) Under nitrogen protection, the improved chiral catalyst (R)-BINAP-RuBr 2 (0.05g) was transferred to a 2L round bottom flask, then degassed methanol (1200mL) and methyl β-carbonyltetradecanoate (III) (R=Me) (200g, 0.78mol) were added, and the reaction temperature was 40°C , the reaction time is 12 hours, and the reaction pressure is 0.1kg / cm 2 ; After the reaction, a brown solution was obtained.
[0023] (2) After replacing the nitrogen in the flask with hydrogen for three times, stir the solution vigorously, keep it warm at 40°C, and keep it at 0.1kg / cm 2 Hydrogenation was carried out at low temperature until the hydrogen was no longer consumed for about 12 hours; after the hydrogen in the flask was removed with nitrogen, the reaction solution was concentrated under reduced pressure, and then recrystallized with n-hexane ...
Embodiment 2
[0024] Example 2 Preparation of (R)-β-hydroxytetradecanoic acid methyl ester, the compound of formula (I) (R=Me)
[0025] Proceed as follows:
[0026] (1) Under nitrogen protection, the improved chiral catalyst (R)-BINAP-RuBr 2 (0.5g) was transferred to a 20L glass reactor, and then degassed methanol (12L) and methyl β-carbonyltetradecanoate (III) (R=Me) (2000g, 7.8mol) were added, and the reaction temperature was 50°C , the reaction time is 10 hours, and the reaction pressure is 0.15kg / cm 2 ; After the reaction, a brown solution was obtained.
[0027] (2) After replacing the nitrogen in the flask with hydrogen for three times, the solution was vigorously stirred, kept at 50°C, and heated at 0.15kg / cm 2 Hydrogenation was carried out at low temperature until the hydrogen was no longer consumed for about 10 hours; after the hydrogen in the flask was removed with nitrogen, the reaction solution was concentrated under reduced pressure, and then recrystallized with n-hexane to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com