New method for separation of phenylalanine enantiomer by reaction extraction
A technology of phenylalanine and enantiomers, which is applied in the field of separation of phenylalanine enantiomers in the reaction extraction process, can solve the problem of low selectivity for separating amino acid enantiomers, achieve good reusability and reduce production costs , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
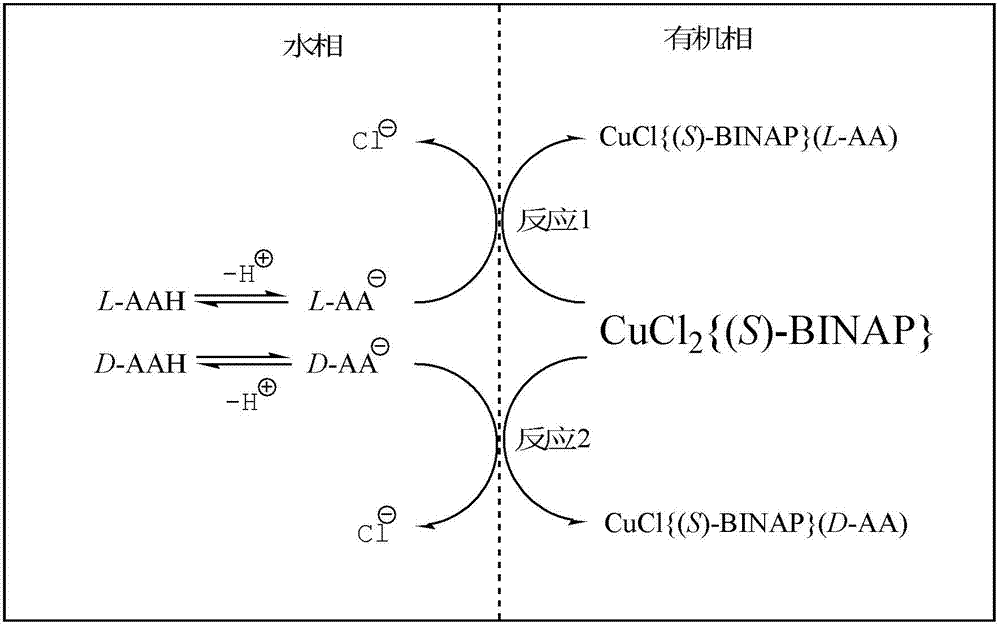
[0011] D,L - Phenylalanine dissolved in 0.1 mmol / L NaH 2 PO 4 / Na 2 HPO 4 Buffer solution and adjust pH = 5.0 ~ 9.0, control D,L - The concentration of phenylalanine is 2~10 mmol / L to obtain the aqueous phase. Chirality ( S )-BINAP and tetraacetonitrile copper hexafluorophosphate ( ), diacetonitrile palladium dichloride and bis(triphenylphosphine) nickel chloride ( ) was dissolved in 1,2-dichloroethane according to the proportion, fully stirred, and controlled ( S )-BINAP and the molar ratio of the aforementioned three metal ions are 0.5 ~ 5, and make ( S )-BINAP concentration was controlled within the concentration range of 1-5 mmol / L to obtain an organic phase. Take 10 mL each of the aqueous phase and the organic phase and put them into test tubes, shake in a constant temperature shaker in a water bath for 9 to 14 hours, and control the constant temperature within the range of 5 to 30 °C. Thereafter, the two phases were kept at constant temperature for more tha...
Embodiment 2
[0013] Tetraacetonitrile copper hexafluorophosphate ( ) as the central ion donor of the extractant, and chiral ( S )-BINAP undergoes complexation reaction in 1,2-dichloroethane to obtain ( S )-BINAP-Copper( ) complex solution, the control solution ( S )-BINAP concentration is 10mmol / L. D,L-phenylalanine dissolved in 0.1mom / L NaH 2 PO 4 / Na 2 HPO 4 Adjust pH = 9.0 in buffer solution, control D,L - The concentration of phenylalanine is 20 mmol / L. An organic phase and an aqueous phase were obtained respectively through the above two steps of operation. Take 50 mL of the organic phase and 50 mL of the aqueous phase in a 250 mL separatory funnel for extraction operation, mix well and let the two phases reach equilibrium. Measure the concentration of phenylalanine enantiomer in organic phase and aqueous phase respectively, and carry out material constant calculation, determine D and L - the partition coefficient of the enantiomer ( k D and k L ) were 0.101 and 0....
Embodiment 3
[0015] Tetraacetonitrile copper hexafluorophosphate ( ) as the central ion donor of the extractant, and chiral ( S )-BINAP undergoes complexation reaction in 1,2-dichloroethane to obtain ( S )-BINAP-Copper( ) complex solution, the control solution ( S )-BINAP with copper ( ) ion concentration is 2 mmol / L. D,L-phenylalanine dissolved in 0.1 mol / L NaH 2 PO 4 / Na 2 HPO 4 buffer solution and adjust to pH = 9.0. Prepare D, L-phenylalanine concentration of 1, 2, 3, 4 mmol / L four groups of aqueous phase solutions. Take 10 mL of the organic phase obtained above and put 10 mL of each of the four groups of water phases into different test tubes, and perform the extraction operation in a constant temperature shaker in a water bath (the constant temperature is controlled at 5°C). Mix well and let stand to separate the layers, so that the two phases reach equilibrium, then detect the extraction effect. The results showed that the partition coefficients of the enantiomers of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com