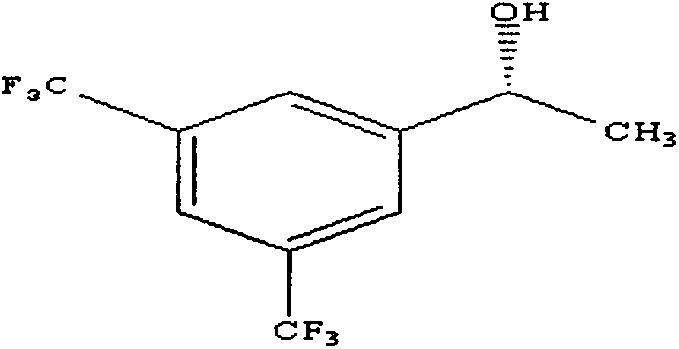
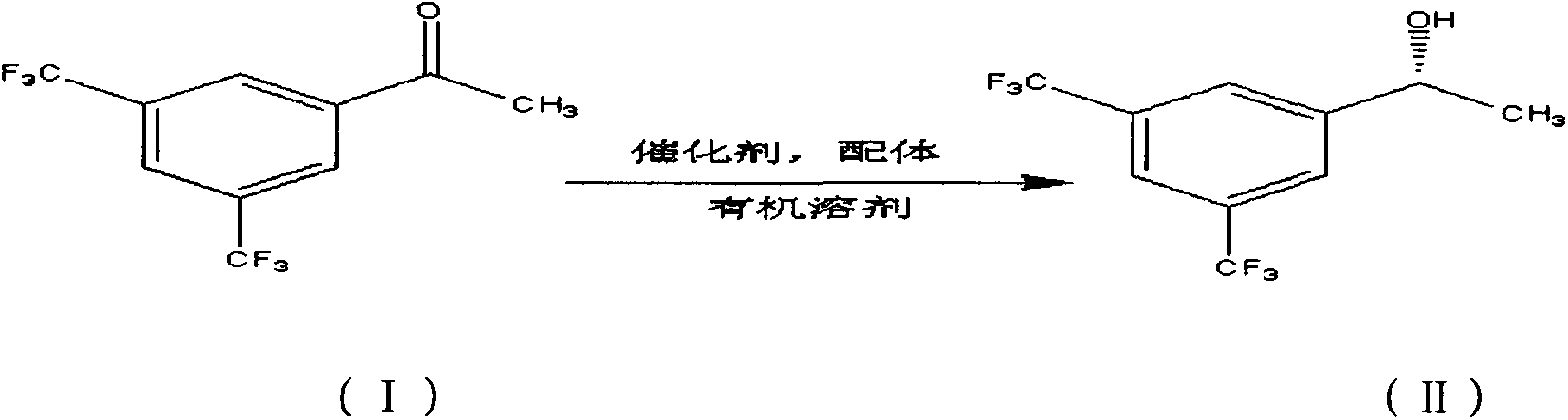
Method for preparing (R)-3,5-bis(trifluoromethyl)benzene-ethanol
A technology of trifluoromethyl and phenethyl alcohol is applied in the field of preparing chiral pharmaceutical intermediates by asymmetric catalytic hydrogenation, and can solve the problems of high production cost, large amount of coenzyme added, low initial concentration and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] Complexation steps:
[0032] 0.0082g catalyst [RuCl 2 (C 10 h 14 ) 2 ] 2 and the chiral phosphine ligand (S,S)-C 6 P 2 (NH) 2 Put it into a (catalyst: ligand = 1:2) 100 mL round bottom flask, add 50 mL of tetrahydrofuran, and stir magnetically at 0° C. for 10 min to fully complex the catalyst and the ligand.
[0033] Reaction steps:
[0034] Add tetrahydrofuran 400mL (organic solvent: substrate = 20: 1) in 2500mL reactor, behind 20mL substrate 3,5-bis (trifluoromethyl) acetophenone and 0.82g KOH (base: catalyst = 100: 1) Put it into the reaction kettle, then add 50 mL of the above-mentioned complexed catalyst and ligand mixed solution, and replace the reaction kettle with hydrogen for 3 times to ensure that the reaction is carried out under anaerobic conditions. Turn on the stirring, set the stirring rate to 600r / min, and the hydrogen pressure to 2MPa. Afterwards, the temperature was raised to 100°C and kept at a constant temperature, and the reaction was carr...
example 2
[0039] Complexation steps:
[0040] 0.0082g catalyst Ir[(COD)Cl] 2 and the chiral ligand (S,S)-C 6 P 2 (NH) 2 (catalyst: ligand = 1: 1) was put into a 100mL round-bottomed flask, 50mL of toluene was added, and magnetically stirred at 10°C under normal pressure for 20min to fully dissolve and complex the catalyst and the ligand;
[0041] Reaction steps:
[0042] In 2500mL reactor, add toluene 2000mL (organic solvent: substrate=100: 1), behind 20mL substrate 3,5-bis(trifluoromethyl) acetophenone and 0.082g KOH (base: catalyst=10: 1) Put it into the reaction kettle, then add 50 mL of the above-mentioned complexed catalyst and ligand mixed solution, and replace the reaction kettle with hydrogen for 3 times to ensure that the reaction is carried out under anaerobic conditions. Stirring was started, the stirring rate was set to 600r / min, and the hydrogen pressure was 2.5MPa. Afterwards, the temperature was raised to 45°C and kept at a constant temperature, and the reaction was...
example 3
[0047] Complexation steps:
[0048] 0.0082g catalyst Ir[(COD)Cl] 2 Put the chiral ligand BINAP (catalyst: ligand = 1: 1.2) into a 100mL round bottom flask, add 50mL of absolute ethanol, and stir magnetically at 20°C for 30min to fully dissolve and complex the catalyst and the ligand;
[0049] Reaction steps:
[0050] In 2500mL reactor, add dehydrated alcohol 600mL (organic solvent: substrate=30: 1), behind 20mL substrate 3,5-bis(trifluoromethyl) acetophenone and 0.164gNaOH (base: catalyst=20 : 1) add in the reactor, then add 50mL of the catalyst and ligand mixed solution that the above-mentioned complexation is completed, replace 3 times with hydrogen in the reactor, to guarantee that the reaction is carried out under anaerobic conditions. Turn on the stirring, set the stirring rate to 600r / min, and the hydrogen pressure to 3MPa. Afterwards, the temperature was raised to 50°C and kept at a constant temperature, and the reaction was carried out for 6h.
[0051] Separation s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com