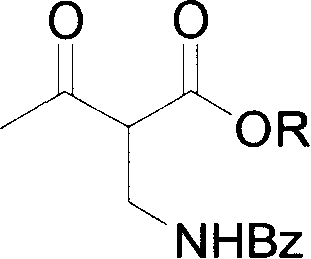
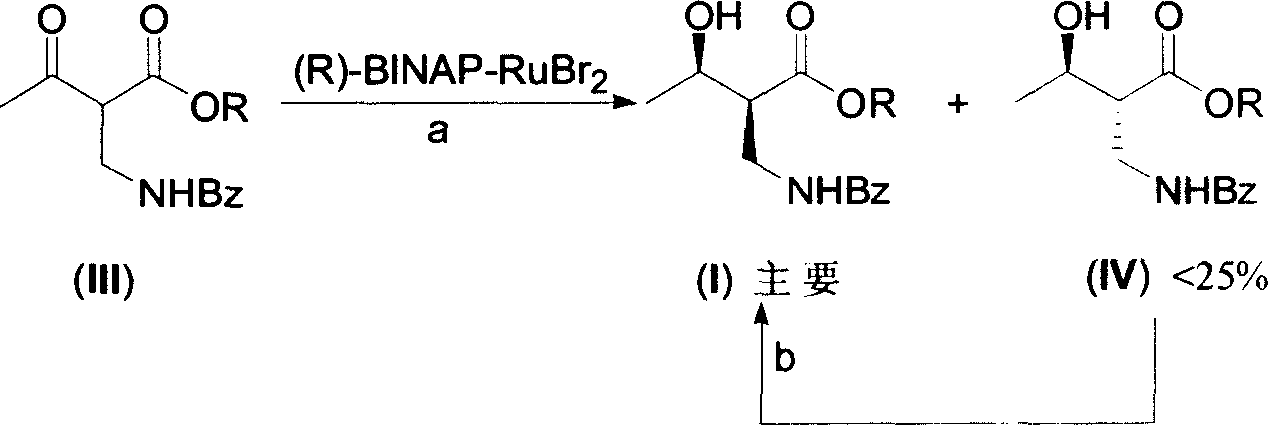
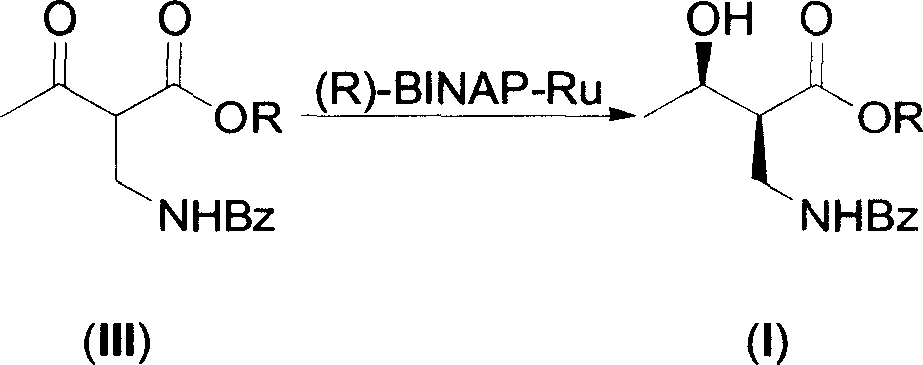Asymmetric catalytic hydrogenation process of synthesizing serial (2S,3R)-2 benzoyl aminomethyl-3-hydroxy butyrate compounds
A technology of benzamidomethyl and hydroxybutyrate, which is applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the limitations of industrial production and synthetic routes Long, cumbersome operation and other problems, to avoid high-pressure hydrogenation equipment, low cost, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The preparation method of the present invention is further described in detail below by way of examples.
[0018] 1. (2S, 3R)-2-benzoylaminomethyl-3-hydroxybutyric acid ethyl ester is the compound of formula (I), (2R, 3R)-2-benzoylaminomethyl-3-hydroxybutyrate Ethyl acid ester is the preparation of formula (IV) compound (R=Et):
[0019] Under nitrogen protection, the improved catalyst (R)-BINAP-RuBr under nitrogen protection 2 (1.0g) was transferred to a 2L round bottom flask, then degassed methanol (1200ml), ethyl 2-benzamidomethyl-3-oxobutanoate (III) (R=Et) (26.3g, 0.1 mol), a brown solution was obtained.
[0020] After replacing the nitrogen in the flask with hydrogen three times, the solution was vigorously stirred at 0.04kg / cm 2 under hydrogenation. To no longer consume hydrogen, about 24 hours. After purging the hydrogen in the flask with nitrogen, the reaction solution was filtered through a silica gel packing layer to remove the catalyst. After the filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com