Method for extraction separation of 4-nitrobenzene glycine enantiomer by multistage centrifugal extractor
A technology of nitrophenylglycine and enantiomers, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of excess enantiomers and low yield, and achieve low extraction inhibition, The effect of simple process and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Dissolve 0.3988 g of 4-nitrophenylglycine enantiomers in 0.1 mol / L of NaH 2 PO 4 / H 3 PO 4 Adjust the pH in the buffer solution to 7.0 to prepare a 1L solution with a concentration of 2 mmol / L as the liquid phase;
[0011] Take 0.6786 g NaPF 6 Soluble in 0.1 mol / L NaH 2 PO 4 / H 3 PO 4 Adjust the pH in the buffer solution to 7.0 to prepare a 2L solution with a concentration of 2 mmol / L as the aqueous phase;
[0012] Take 0.7685 g [(CH 3 EN) 4 Cu]PF 6 and 1.2579 g BINAP were dissolved in pure 1,2-dichloroethane organic solvent, magnetically stirred, and reacted in the dark for 12 hours, and a 2L solution was prepared as the organic phase;
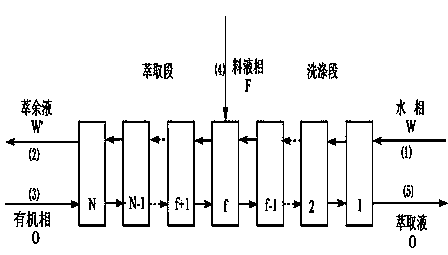
[0013] Connect 10-stage centrifugal extractors in series, use a centrifugal pump to pump the organic phase into the centrifugal extractor first, when the organic phase flows out from the outlet of the extraction phase, pump the water phase into the centrifugal extractor from the corresponding inlet, and then enter the equili...
Embodiment 2
[0015] Dissolve 0.3988 g of 4-nitrophenylglycine enantiomers in 0.1 mol / L of NaH 2 PO 4 / H 3 PO 4 Adjust the pH in the buffer solution to 7.0 to prepare a 1L solution with a concentration of 2 mmol / L as the liquid phase;
[0016] Take 0.6786 g NaPF 6 Soluble in 0.1 mol / L NaH 2 PO 4 / H 3 PO 4 Adjust the pH in the buffer solution to 7.0 to prepare a 2L solution with a concentration of 2 mmol / L as the aqueous phase;
[0017] Take 0.7685 g [(CH 3 EN) 4 Cu]PF 6 and 1.2579 g BINAP were dissolved in pure 1,2-dichloroethane organic solvent, magnetically stirred, and reacted in the dark for 12 hours, and 2 L was dissolved in as the organic phase;
[0018] Connect 14 stages of centrifugal extractors in series, use a centrifugal pump to pump the organic phase into the centrifugal extractor first, when the organic phase flows out from the outlet of the extraction phase, pump the water phase into the centrifugal extractor from the corresponding inlet, and then enter the equilibr...
Embodiment 3
[0020] Take 0.7976 g of 4-nitrophenylglycine enantiomers dissolved in 0.1 mol / L of NaH 2 PO 4 / H 3 PO 4 Adjust the pH in the buffer solution to 7.0 to prepare a 1L solution with a concentration of 4 mmol / L as the liquid phase;
[0021] Take 1.3572 g NaPF 6 Soluble in 0.1 mol / L NaH 2 PO 4 / H 3 PO 4 Adjust the pH in the buffer solution to 7.0 to form a 2 L solution with a concentration of 4 mmol / L as the aqueous phase;
[0022] Take 1.5370 g [(CH 3 EN) 4 Cu]PF 6 and 2.5158 g of BINAP were dissolved in pure 1,2-dichloroethane organic solvent, stirred by magnetic force, and reacted in the dark for 12 hours, and made into 2L and dissolved in the organic phase;
[0023]Connect 14 stages of centrifugal extractors in series, use a centrifugal pump to pump the organic phase into the centrifugal extractor first, when the organic phase flows out from the outlet of the extract phase, pump the water phase into the centrifugal extractor from the corresponding inlet, and then ente...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com