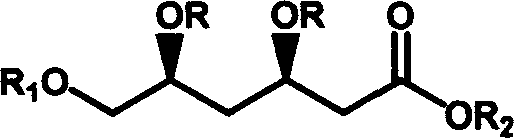
Method for preparing t-butyl (3R, 5S)-3,5,6-trihydroxy-hexanoate
A technology of tert-butyl hexanoate and ethyl benzyloxybutyrate, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of low production rate and low production rate, and achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
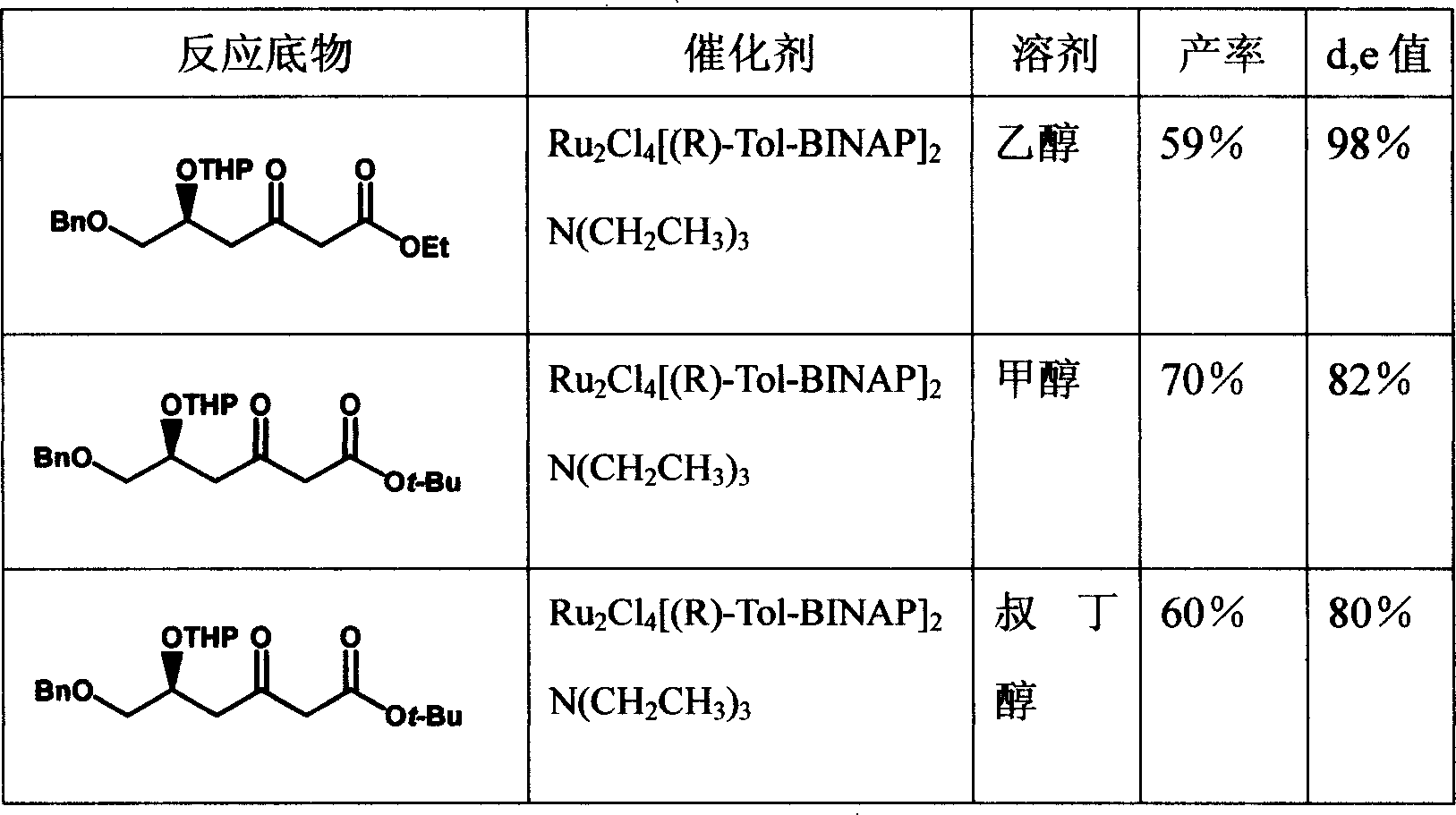
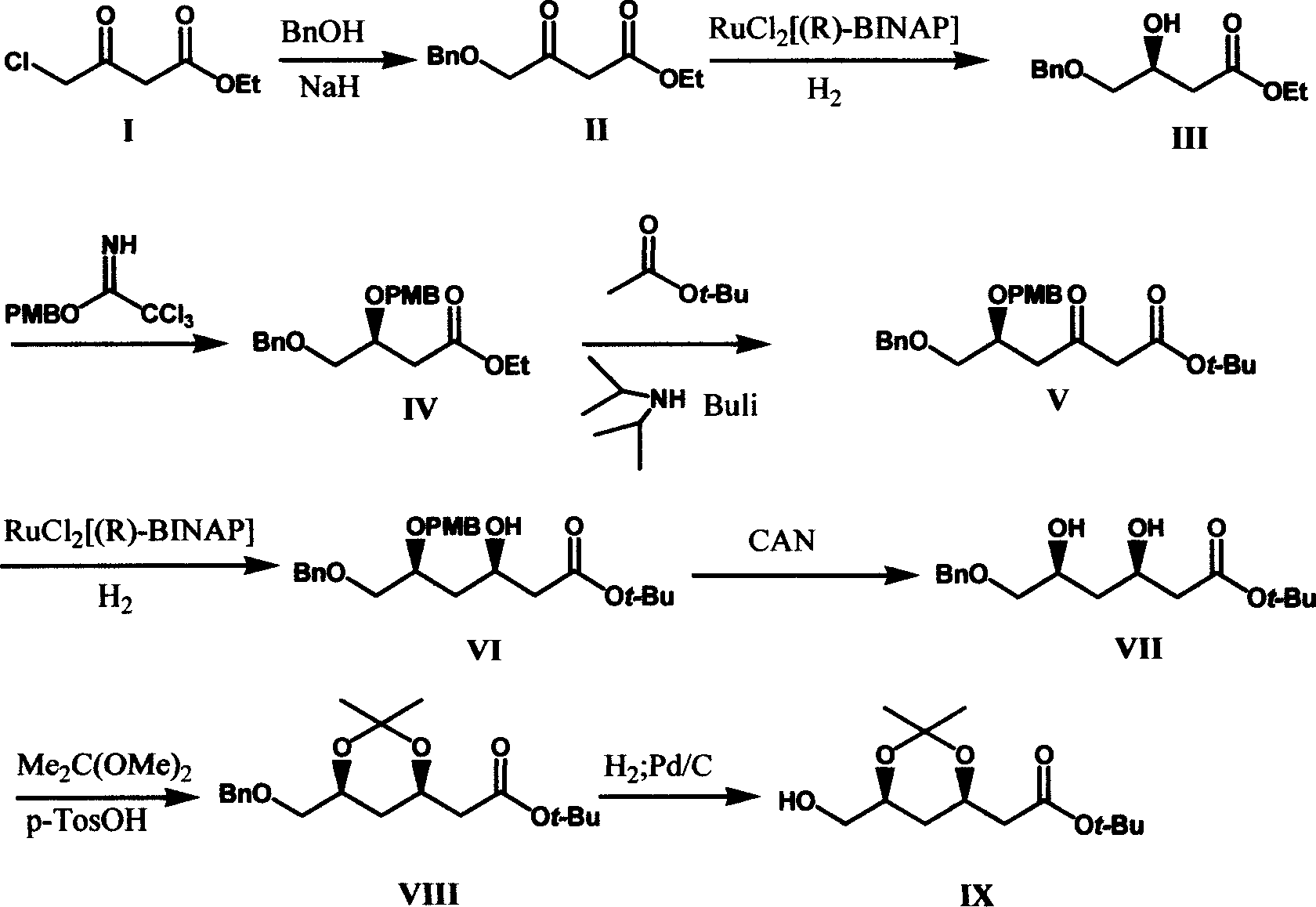
[0024] The first step of preparation of ethyl benzyloxyacetoacetate (II)
[0025] 7.35g (55%-60%) of sodium hydride (168-184mmol) was suspended in 100ml of toluene, cooled in an ice bath, and 16.2g of benzyl alcohol (15.5ml, 150mmol) was slowly dropped into it within 30 minutes. After stirring at room temperature for 2 hours, a white slurry formed. After cooling in an ice bath, 12.5 g of ethyl chloroacetoacetate (76 mmol) was dropped into it, and the reaction was carried out overnight at room temperature for 19 hours. Add 75.5ml of 2N citric acid, separate the layers, extract the aqueous phase with toluene twice, combine the organic phases, dry, rotary evaporate, and wash the residue with (2×10ml) heptane. The residue was distilled under reduced pressure to obtain 15 g of ethyl benzyloxyacetoacetate (II), with a yield of 84%.
[0026] The second step (3S) - preparation of ethyl 3-hydroxy-4-benzyloxybutyrate (III)
[0027] Catalyst preparation: 36mg (R)-(+)-BINAP, 14mg dipol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com