Process for the purification of Montelukast
A purification method, the technology of montelukast, applied in the field of preparation of high-purity montelukast, can solve the problems of long-term cycle, unfavorable economy, troublesome purification, etc., and achieve the effect of high yield and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
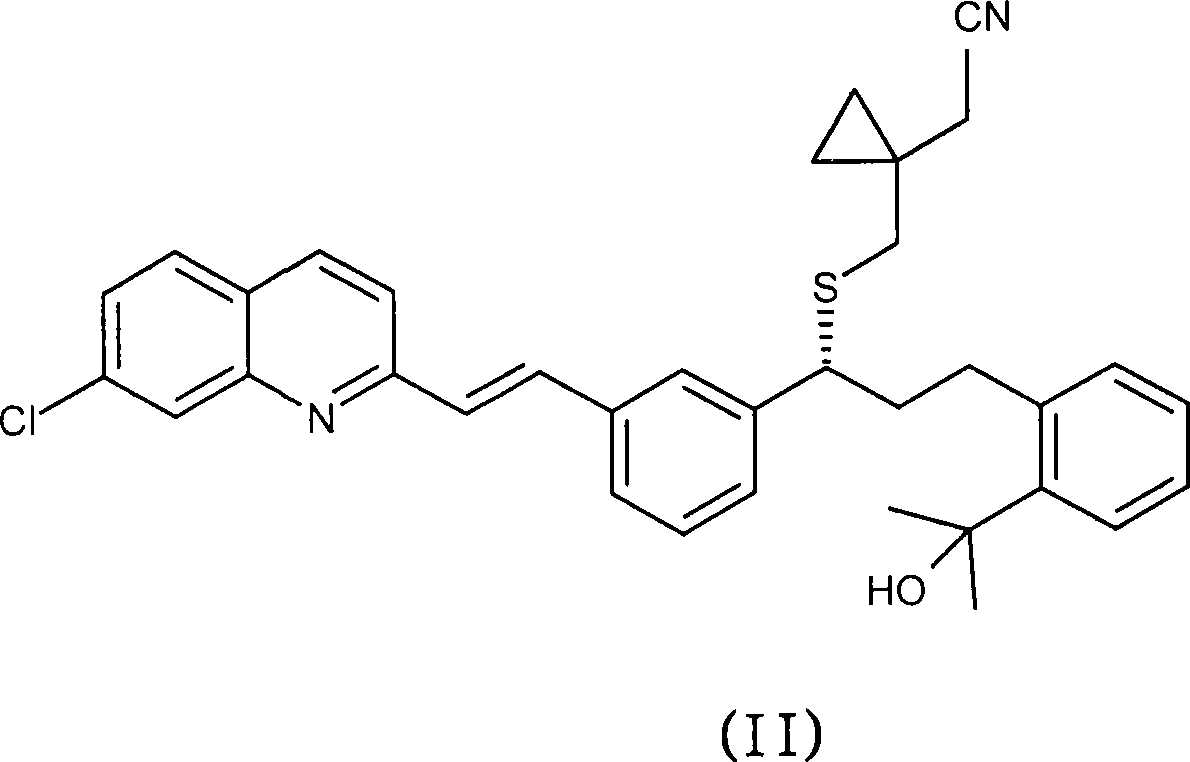
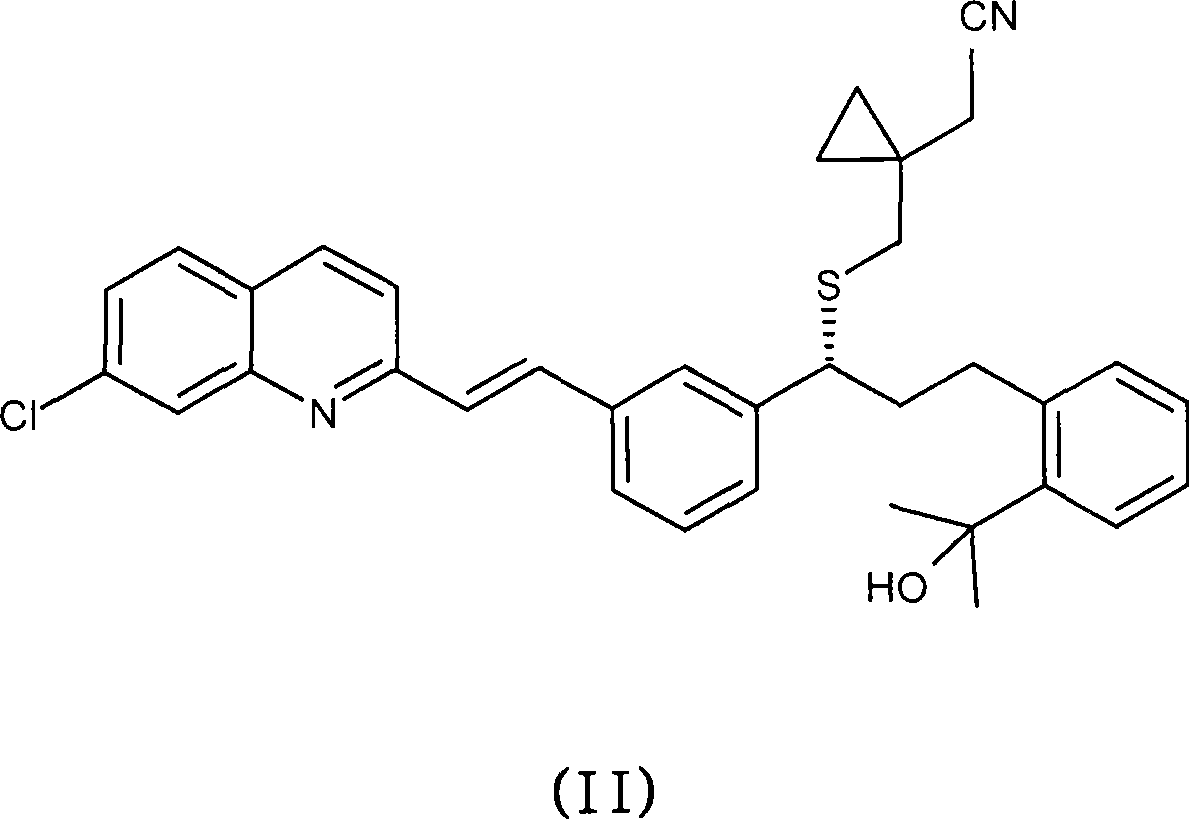
[0037] Example 1 : Preparation of montelukast acid
[0038] 143.6 g of sodium hydroxide was added to 102 g of (R, E)-2-(1-((1-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3 - (2-(2-Hydroxypropan-2-yl)phenyl)propylthio)methyl)cyclopropyl)acetonitrile in 407ml ethanol 96% (v / v) solution. The mixture was stirred at reflux temperature for 30 hours. After this period, the solvent was distilled off in vacuo (purification by HPLC: 61.5 area %; impurity i 2 : 1.84 area%; impurity i 5 : 3.70 area%; impurity i 6 : 1.42 area %). The mixture was partitioned between 1000 ml toluene and 1500 ml water at room temperature. The aqueous phase in which the inorganic salts were dissolved was discarded. The organic phase was mixed with 1500 ml of water and the mixture was heated to 60° C. and stirred for 15 minutes. At this point, the product dissolved in the aqueous layer. The pH of the aqueous layer was 12.5. Some impurities from the preparation process were dissolved in the organic la...
Embodiment 2
[0039] Example 2 : Crystals of montelukast acid
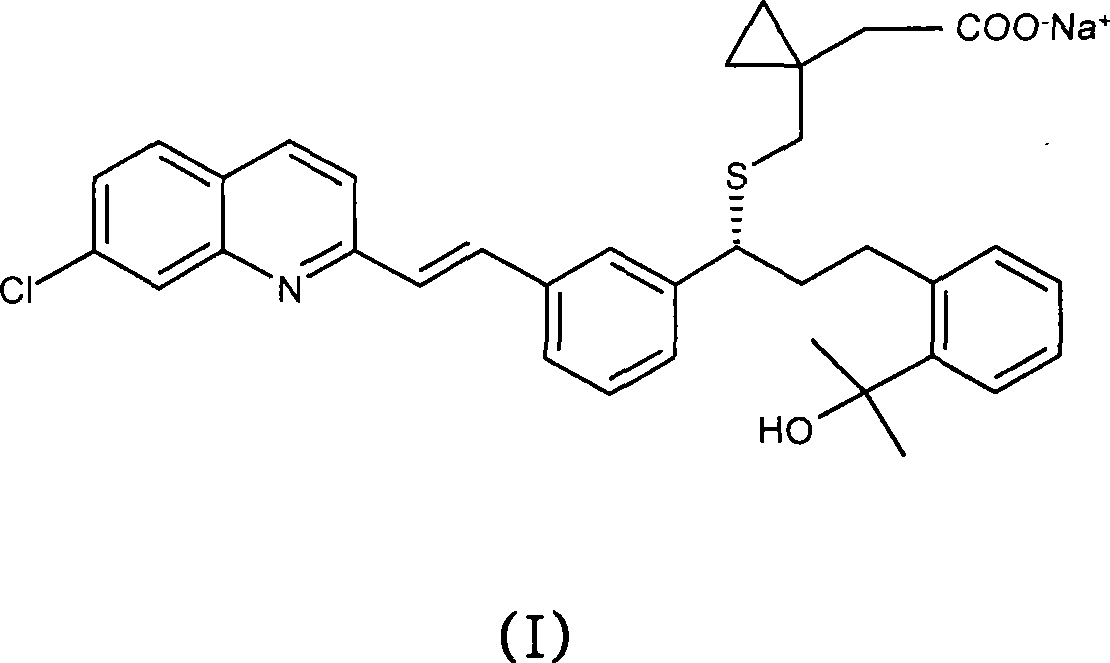
[0040] The toluene solution from the previous example was stirred at room temperature for 12 hours. After this time, a yellow suspension had formed. The resulting solid was filtered off, washed with toluene and dried under vacuum at 30 °C for 24 hours. 53 g of montelukast acid were recovered (purification by HPLC: 96 area %). Yield: 90%.
Embodiment 3
[0041] Example 3 : Purification of montelukast acid using toluene as extraction solvent
[0042] 45 ml of 0.5M aqueous NaOH was added to montelukast acid (impurity i 2 : 0.05 area%; impurity i 3 : 1.39 area%; impurity i 5 : 0.20 area%; impurity i 6 : 0.05 area %) and 30ml suspension of toluene. A bilayer solution was formed in which montelukast was dissolved as the sodium salt in the aqueous layer. After stirring at 40°C for 30 minutes, the organic layer was discarded. Wash with 30 ml of toluene two more times in sequence, each time adjusting the pH to 12.2-13.2. The resulting aqueous solution was acidified to pH 9.3 with 2M aqueous acetic acid and washed twice with 30 ml of toluene. Both extractions were performed at 60°C. Finally, another 30 ml portion of toluene was added to the aqueous solution and the mixture was acidified to pH 6.0 with 2M aqueous acetic acid at room temperature. The final organic layer was separated and kept as a solution of purified montelu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com