Method for preparing 7-chloroquinaldine by use of phase-transfer catalytic reaction
A phase transfer catalysis and reaction technology, applied in the direction of organic chemistry, etc., can solve the problems of harsh process conditions, product loss, high price of tetrachloro-p-benzoquinone, and achieve the effects of improving reaction efficiency, increasing emulsification, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
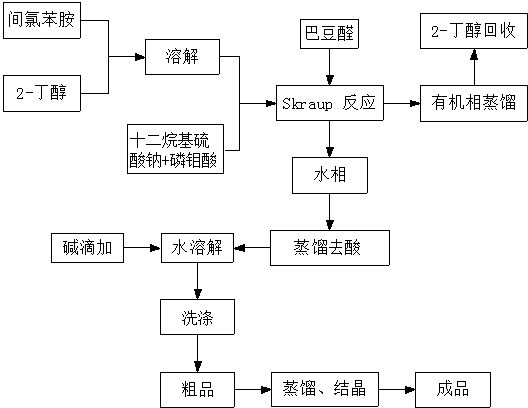
[0024] First dissolve 12.7g m-chloroaniline in 50mL of organic solvent 2-butanol, add it to a three-necked flask and stir, add 14.4g of surfactant sodium lauryl sulfate and 18.25g of oxidant phosphomolybdic acid, add 100mL of aqueous solution and The mixed solvent of 2-butanol was stirred at 80 °C for 1 h; then 7 g of crotonaldehyde was added dropwise to the mixture, and the reaction was continued at 80 °C for 4 h; after the reaction, the mixture was layered, and 2-butanol was recovered from the organic phase , the aqueous phase was distilled under reduced pressure to remove acid, and the 3 The solution was washed and recrystallized from ethanol to obtain high-purity 7-chloroquinaldine with a yield of 85%.
Embodiment 2
[0026] First dissolve 12.7g m-chloroaniline in 50mL of organic solvent 2-butanol, add it to a three-necked flask and stir, add 28.8g of surfactant sodium lauryl sulfate and 36.5g of oxidant phosphomolybdic acid, add 100mL of aqueous solution and The mixed solvent of 2-butanol was stirred at 85°C for 2h; then 7g of crotonaldehyde was added dropwise to the mixture, and the reaction was continued at 85°C for 3h; after the reaction, the mixture was layered, and 2-butanol was recovered from the organic phase , the aqueous phase was distilled under reduced pressure to remove the acid, and the aqueous phase was distilled off with 8wt% 100mL NaHCO 3 The solution was washed and recrystallized with ethanol to obtain high-purity 7-chloroquinaldine with a yield of 83%.
Embodiment 3
[0028] First dissolve 12.7g m-chloroaniline in 50mL of organic solvent 2-butanol, add it to a three-necked flask and stir, add 20.4g of surfactant sodium lauryl sulfate and 30.5g of oxidant phosphomolybdic acid, add 100mL of aqueous solution and The mixed solvent of 2-butanol was stirred at 90°C for 2h; then 7g of crotonaldehyde was added dropwise to the mixture, and the reaction was continued at 90°C for 4h; after the reaction, the mixture was layered, and 2-butanol was recovered from the organic phase , the aqueous phase was distilled under reduced pressure to remove the acid, and the aqueous phase was distilled off with 8wt% 100mL NaHCO 3 The solution was washed and recrystallized with ethanol to obtain high-purity 7-chloroquinalidine with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com