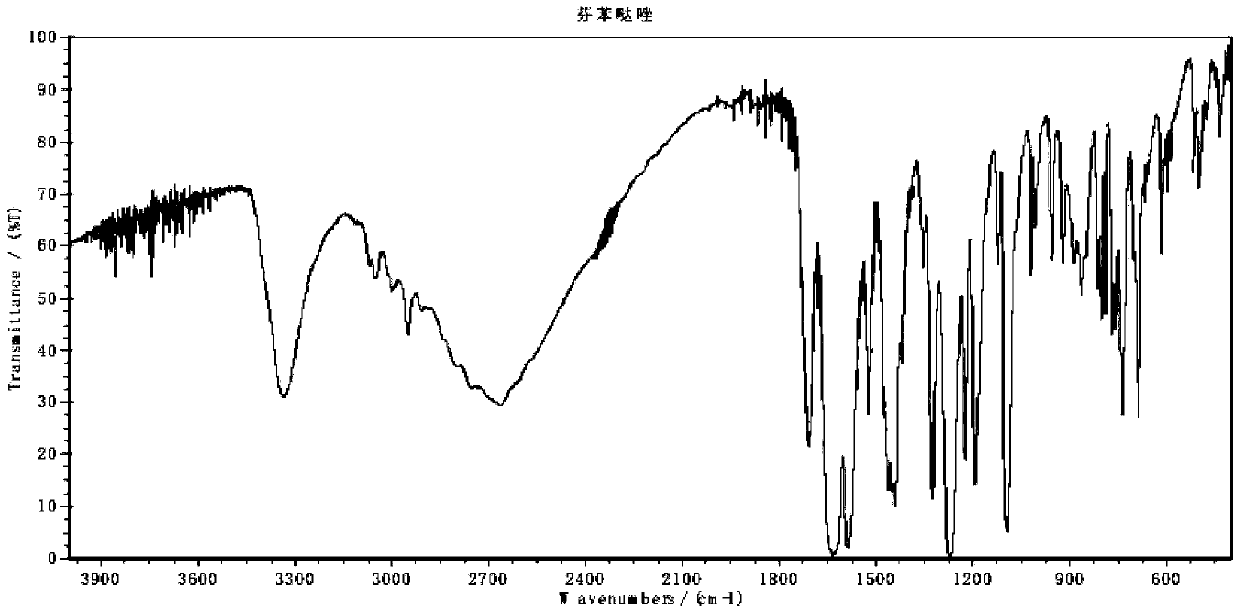
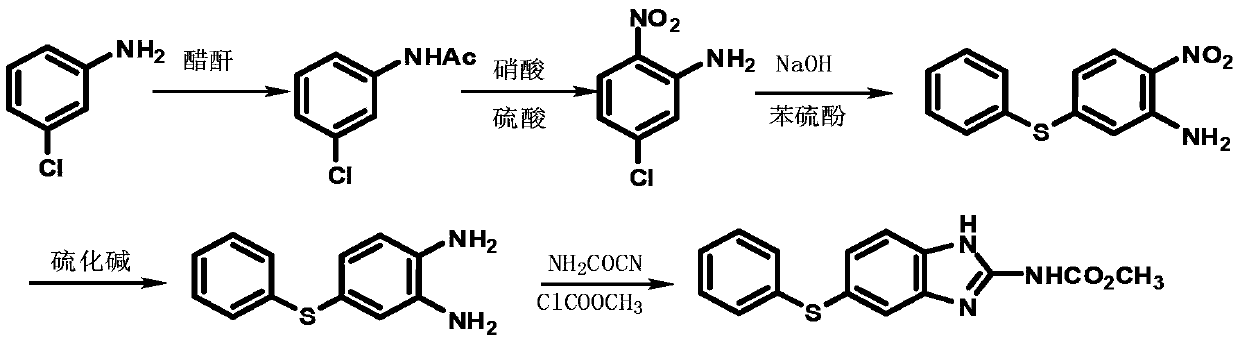
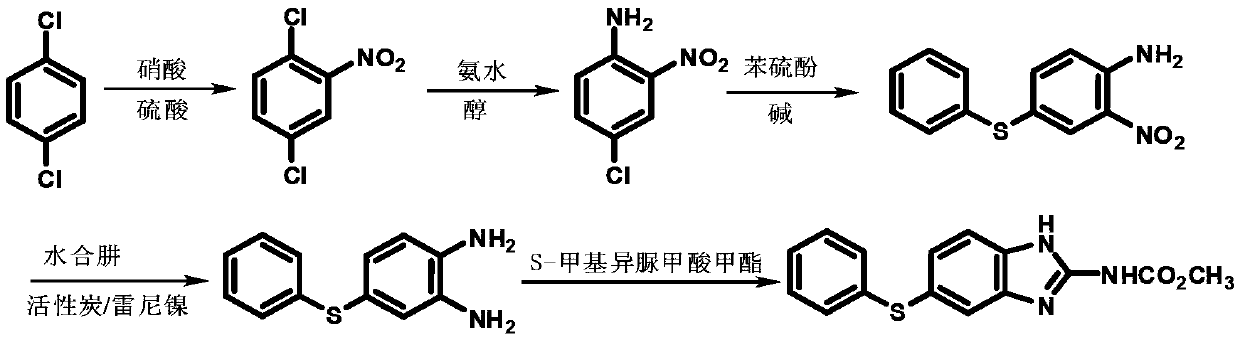
A kind of preparation method of fenbendazole
A technique for preparing fenbendazole and its preparation steps, which is applied in the field of preparation of fenbendazole, can solve problems such as low condensation yield, and achieve the effects of high quality, simple and efficient synthesis process, and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 12, the preparation of 4-dichloronitrobenzene
[0034] In a 500ml four-necked flask with a thermometer and a stirring device, start stirring, add 100.0g m-dichlorobenzene, 0.5 times the weight ratio of concentrated sulfuric acid, drop nitric acid in a molar amount that is 1.05 times m-dichlorobenzene, and control the dropping temperature at 10-15°C, after the dropwise addition is completed, keep the temperature at 10-15°C for 3 hours, follow the reaction by HPLC, remove the lower waste acid layer, add 100g of 15% sodium carbonate aqueous solution, stir and wash, and separate the lower layer to obtain 2,4- Dichloronitrobenzene 127.0g was directly used in the next step reaction. This step yield is 97.2%, and content is 98.1%.
[0035] Preparation of step 22-chloro-4-phenylthionitrobenzene
[0036] In a 500ml four-necked flask with a thermometer and a stirring device, start stirring, add 90.8g of 2,4-dichloronitrobenzene, 1.02 times the molar amount of thiophenol, a...
Embodiment 2
[0045] Step 12, the preparation of 4-dichloronitrobenzene
[0046] In a 500ml four-neck flask equipped with a thermometer and a stirring device, start stirring, add 100.0g m-dichlorobenzene, 0.9 times the weight ratio of concentrated sulfuric acid, and the molar amount is 1.1 times m-dichlorobenzene nitric acid, and keep warm at 5-10°C After 3 hours, HPLC traced the reaction to be complete. The lower waste acid layer was separated, and 100 g of 5% sodium hydroxide aqueous solution was added to stir and wash. The lower layer was separated to obtain 125.0 g of 2,4-dichloronitrobenzene, which was directly used in the next reaction. This step yield is 95.7%, and content is 98.7%.
[0047] Preparation of step 22-chloro-4-phenylthionitrobenzene
[0048] In a 500ml four-necked flask with a thermometer and a stirring device, start stirring, add 90.8g of 2,4-dichloronitrobenzene, 1.5 times the molar amount of thiophenol, and 3 times the amount of 2,4-dichloronitronitrobenzene Toluene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com