Synthesis method of 7-chloroquinaldine
A synthetic method, the technology of chloroquine, which is applied in the field of synthesis of 7-chloroquinaldine, achieves the effect of strong market competitiveness, good stability and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
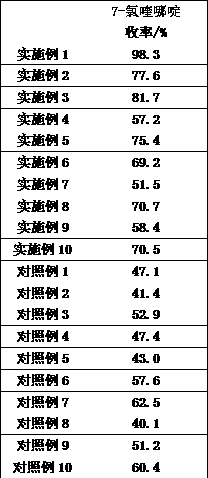
Examples
Embodiment 1
[0016] A kind of synthetic method of 7-chloroquinaldine is characterized in that the method comprises the following steps:
[0017] Step 1. Install a thermometer, a dropping funnel and a reflux condenser on a 500 ml three-necked bottle. Add o-nitrotoluene 34.0g, 3.4gSiO2 to the bottle 2 -HEPIMBr catalyst and mass fraction 30% hydrochloric acid 240ml;
[0018] Step 2. After mixing evenly, slowly add m-chloroaniline 104.0g and heat up to 100°C, slowly drop 68.0g crotonaldehyde into the system, after 5h, after reflux at reflux temperature for 4h, steam distillation to remove o-nitrotoluene and dilute hydrochloric acid;
[0019] Step 3, the residual liquid in the bottle is neutralized with ammonia water with a mass fraction of 28% to pH = 7, and the activated carbon removes impurities to obtain an oily liquid;
[0020] Step 4. Distill the above-mentioned oily liquid under reduced pressure to collect oily fractions at 175-185° C. / 5.5 kPa, which become solid after cooling, and re...
Embodiment 2
[0027] Step 2. After mixing evenly, slowly add m-chloroaniline 94.0g and raise the temperature to 100°C, slowly add 68.0g crotonaldehyde into the system, after 5h, after reflux at reflux temperature for 4h, steam distillation to remove o-nitrotoluene And dilute hydrochloric acid; All the other steps are with embodiment 1.
Embodiment 3
[0029] Step 2. After mixing evenly, slowly add 84.0g of m-chloroaniline dropwise and raise the temperature to 100°C, slowly add 68.0g of crotonaldehyde into the system dropwise, after 5h, reflux at reflux temperature for 4h, steam distillation to remove o-nitrotoluene And dilute hydrochloric acid; All the other steps are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com