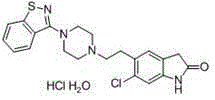
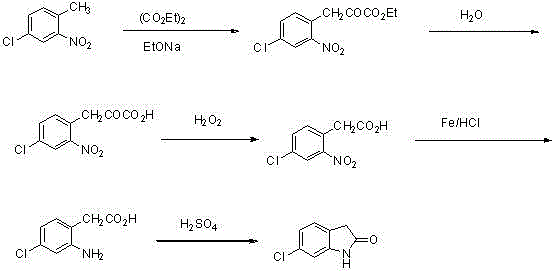
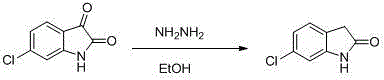The synthetic method of ziprasidone intermediate
A synthesis method and technology of ziprasidone, applied in the field of medicine, can solve the problems of high toxicity, difficult to obtain raw materials and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: compound II reduction preparation compound III
[0027] Example 1-1 Add 200 mL of water to the reaction flask, heat to 40°C, add 17.0 g (143 mmol) of tin powder and adjust the pH to 3 with concentrated hydrochloric acid, continue to heat up to 60°C, add 9.0 g (57.1 mmol) and 23.9 g (201 mmol) of tin powder, after adding, react at 60~80°C for 8 hours, then add 10% sodium hydroxide solution, adjust the pH to 8~9, and stir for 30 minutes. After suction filtration, the filtrate was acidified with concentrated hydrochloric acid to pH 4~5, and a yellow solid precipitated out. The solid was collected and vacuum-dried to obtain 6.7 g of compound IV yellow solid, with a yield of 91.2%.
[0028] Example 1-2 Add 150 mL of water to the reaction flask, heat to 40°C, add 9.3 g (143 mmol) of zinc powder and 150 mL of acetic acid, continue to heat up to 60°C, add 9.0 g (57.1 mmol) of compound II in batches ) and 13.1 g (201 mmol) of iron powder, after adding, react at 60...
Embodiment 2
[0036] Embodiment 2: Preparation of compound IV by acylation of compound III
Embodiment 2-1
[0037] Example 2-1 Dissolve 10.2 g (80 mmol) of compound III and 13.2 mL (96 mmol) of triethylamine in 80 mL of dichloromethane, cool to 0°C, and slowly add 7.6 mL (96 mmol) of chloroacetyl chloride dropwise , After the dropwise addition, return to 25°C and stir for 20h. Evaporate to dryness under reduced pressure, wash the solid with water (80 mL×3), and suction filter to obtain a white solid, which was further recrystallized with ether to obtain 15.7 g of compound IV as a white solid, with a yield of 96.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com