Synthesis method of m-fluoroaniline
A synthesis method, m-fluoroaniline technology, applied in the field of m-fluoroaniline synthesis, can solve the problems of high cost, low yield of Schiemann reaction, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
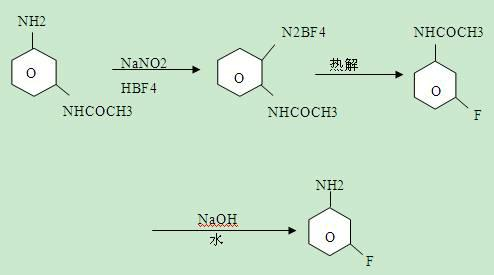
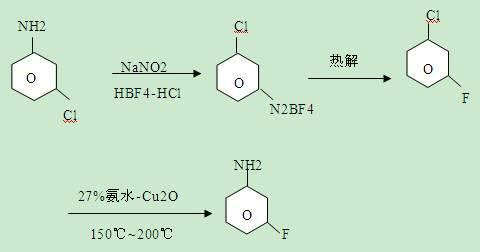
[0023] The reaction equation for synthesizing m-fluoroaniline from m-chloroaniline is:
[0024]
[0025] (1) Preparation of m-fluorochlorobenzene: that is, diazotization reaction followed by thermal decomposition reaction to prepare m-fluorochlorobenzene:
[0026] Put 140g of water into a 1000ml four-necked reaction flask, add 270g of hydrochloric acid with a concentration of 30%, slowly add 128g of m-chloroaniline after stirring evenly, cool to -10°C-0°C after adding, add dropwise 30% of hydrochloric acid Sodium nitrite solution 250g, after adding -10°C-0°C, react for 20 minutes, then add dropwise 280g of 40% fluoboric acid at -10°C-0°C, add -10°C-0°C, react for 1 hour, filter, Dry to obtain diazonium fluoroborate, then thermally decompose the diazonium fluoroborate at 150°C-200°C, add water to neutralize and separate to obtain 90.5g of m-fluorochlorobenzene, 99% or more in GC analysis, yield 71 %;
[0027] (2) Preparation of m-fluoroaniline:
[0028] In the 1000ml auto...
Embodiment 2
[0030] (1) The preparation of m-fluorochlorobenzene is the same as step (1) of implementation 1;
[0031] (2) Preparation of m-fluoroaniline:
[0032] Substitute the Cu of embodiment 1 step (2) with CuO 2 O is the catalyst, the amount is the same, and the rest are the same as in Example 1 to obtain 91 g of m-fluoroaniline, with a yield of 82% and a content of >99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com