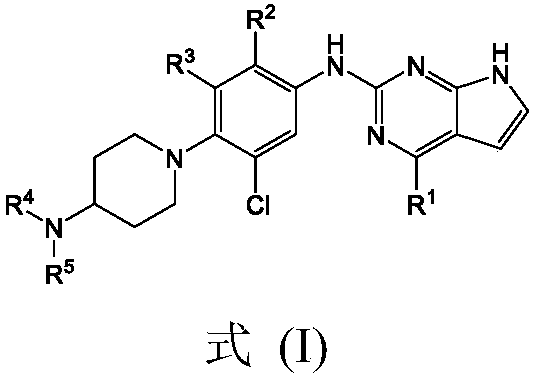
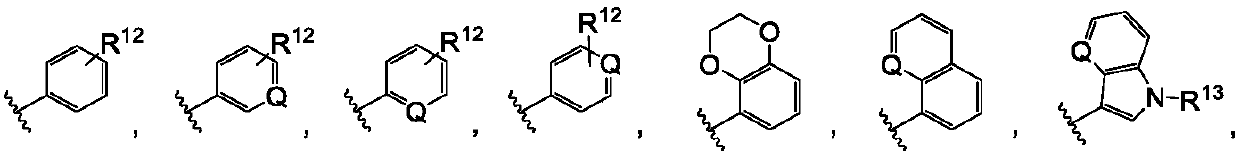
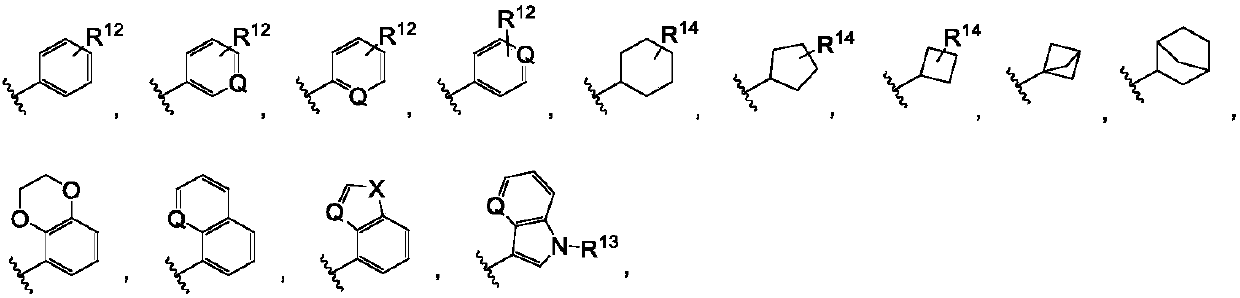
Pyrrolopyrimidine compounds containing m-chloroaniline substituents as well as preparation method and application of pyrrolopyrimidine compounds
A compound and hydrate technology, applied in the field of medicine, can solve the problems of reduced curative effect, drug failure, loss of Cys797 covalent binding, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1. (2-((2-((5-chloro-4-(4-(dimethylamino)piperidin-1-yl)-2-methoxyphenyl)amino)-7H-pyrrole Preparation of [2,3-d]pyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide
[0150]
[0151] Step 1) (2-((2-((5-chloro-4-(4-(dimethylamino)piperidin-1-yl)-2-methoxyphenyl)amino)-7-(( Preparation of 2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide
[0152]280 mg (1 mmol) of 1-(4-amino-2-chloro-5-methoxyphenyl)-N,N-dimethylpiperidin-4-amine, (2-((2-chloro-7 -((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)phenyl)phosphine dimethyl 450 mg ( 1 mmol), 17 mg (0.1 mmol) of p-toluenesulfonic acid were placed in a reaction flask, 10 ml of sec-butanol was added, heated and stirred until the reaction was completed, and 350 mg of the product was obtained by column chromatography after rotary evaporation and concentration, with a yield of 50%. MS:698[M+H] + .
[0153] Step 2) (2-((2-((5-chloro-4-(4-(di...
Embodiment 2
[0155] Example 2. (2-((2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) Preparation of amino)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide
[0156]
[0157] The preparation of reference example 1, in the starting material with equimolar equivalent of 5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) Aniline was substituted for 1-(4-amino-2-chloro-5-methoxyphenyl)-N,N-dimethylpiperidin-4-amine. 1 H NMR(400MHz,DMSO-d6)δ11.62(s,1H),11.35(s,1H),8.97–8.76(m,1H),8.36(s,1H),7.63–7.48(m,2H), 7.46(s,1H),7.08(t,J=7.7Hz,1H),6.96(t,J=2.8Hz,1H),6.82(s,1H),6.38(d,J=2.7Hz,1H), 3.89(s,3H),3.28–3.23(m,2H),2.68(t,J=11.4Hz,2H),2.59–2.51(m,4H),2.42–2.23(m,5H),2.16(s, 3H),1.91–1.85(m,2H),1.83(d,J=13.4Hz,6H),1.68–1.49(m,2H); MS:623[M+H] + .
Embodiment 3
[0158] Example 3. N-(5-chloro-4-(4-(dimethylamino)piperidin-1-yl)-2-methoxyphenyl)-4-(1-methyl-1H-ind Preparation of Indol-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine
[0159]
[0160] With reference to the preparation of Example 1, in the starting material, equimolar equivalents of 2-chloro-4-(1-methyl-1H-indol-3-yl)-7-((2-(trimethylsilyl) )ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine instead of (2-((2-chloro-7-((2-(trimethylsilyl)ethoxy)methyl )-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)phenyl)phosphonium dimethyl. 1 HNMR(400MHz,DMSO-d6)δ11.52(s,1H),8.65(d,J=7.9Hz,1H),8.51(s,1H),8.41(s,1H),7.60(s,1H), 7.53(d, J=8.1Hz, 1H), 7.28(t, J=7.5Hz, 1H), 7.20(t, J=6.6Hz, 2H), 6.86(d, J=9.0Hz, 2H), 3.94( d,J=8.1Hz,6H),3.34–3.31(m,2H),2.70(t,J=11.1Hz,2H),2.28–2.25(m,1H),2.24(s,6H),1.94–1.78 (m,2H),1.67–1.51(m,2H); MS:530[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com