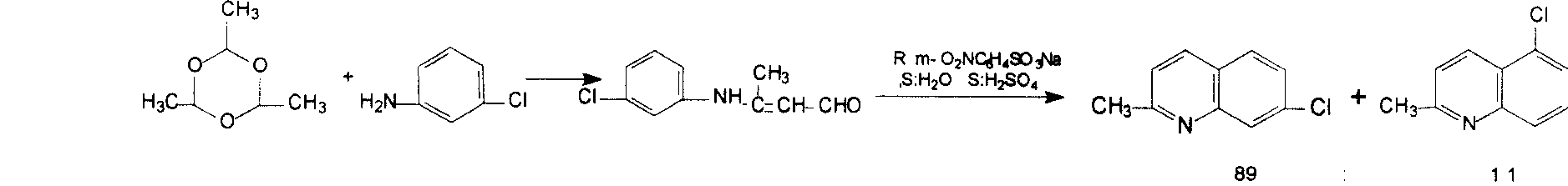Preparation method of midbody 7-chloroquinaldine
An intermediate, the technology of chloroquine, which is applied in the field of preparation of 7-chloroquinaldine, can solve the problems of no solution for product separation and purification, and no practical application value, so as to achieve a safe and reliable reaction process and suppress a large number of isomers Produce, reduce the effect of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of m-chloroaniline hydrobromide:
[0030] In 1000 milliliters of four-necked flasks with mechanical stirring, thermometer, condensing tube, dropping funnel, add hydrobromic acid (500 grams, 40%, 2.5mol, commercially available industrial grade), cooling with water bath, start to drop m-chlorine 292 grams of aniline, 99%, 2.273mol, commercially available industrial grade), the control temperature is not more than 50 degrees, and the dropwise addition time is about 30 minutes. After cooling to room temperature, begin to filter to obtain m-chloroaniline hydrobromide, and dry to obtain 454 grams, content 99%, yield 95%.
[0031] In 2000 milliliters of four-necked flasks with mechanical stirring, thermometer, condenser, dropping funnel, add the hydrobromide (211g, 1mol) of m-chloroaniline, 1000 milliliters of methyl alcohol, 300 milliliters of o-nitrotoluene, heat up and reflux, Add dropwise crotonaldehyde (trans commercially available 85g, 98%, 1.2mol) for a...
Embodiment 2
[0034] In a 2000 ml four-necked flask with mechanical stirring, a thermometer, a condenser, and a dropping funnel, add the hydrobromide (211g, 1mol) of m-chloroaniline, 1000 ml of methanol, 300 ml of toluene, and 100 ml of o-nitrotoluene , warming up to reflux, dropwise adding crotonaldehyde (trans-commercially available 85g, 98%, 1.2mol) for about 20 minutes, refluxed for 40 minutes, cooled to 15-25 degrees, white solids were precipitated, filtered, methanol 2×200 Milliliter washing, 201 grams of 7-chloroquinaldine hydrobromide was obtained, the content was 99.6%, and the yield was 77.0%. 201 grams (0.77mol) of hydrobromide was added in 410 milliliters of 10% sodium carbonate solution, stirred for 30 minutes, and filtered to obtain 136.8 grams of white solid 7-chloroquinaldine, with a content of 99.5% and a yield of 100 %, filter the mother liquor and add 2000 milliliters of water, separate 390 milliliters of the organic layer for the next reaction, and the water layer is dir...
Embodiment 3
[0036] In a 2000 ml four-neck flask with mechanical stirring, a thermometer, a condenser tube, and a dropping funnel, add the hydrobromide (211g, 1mol) of m-chloroaniline, 1000 ml of methanol, 200 ml of toluene, and 200 ml of o-nitrotoluene , warming up to reflux, dropwise adding crotonaldehyde (trans-commercially available 85g, 98%, 1.2mol) for about 20 minutes, refluxed for 40 minutes, cooled to 15-25 degrees, white solids were precipitated, filtered, methanol 2×200 Milliliter washing, 7-chloroquinaldine hydrobromide 198 grams, content 99.6%, yield 76.3.0%. 198 grams (0.76mol) of hydrobromide was added in 410 milliliters of 10% sodium carbonate solution, stirred for 30 minutes, filtered to obtain 135 grams of white solid 7-chloroquinaldine, content 99.4%, yield 100 %, filter the mother liquor and add 2000 milliliters of water, separate 385 milliliters of the organic layer for the next reaction, the water layer is directly recovered by methanol distillation.
[0037]In 2000 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com