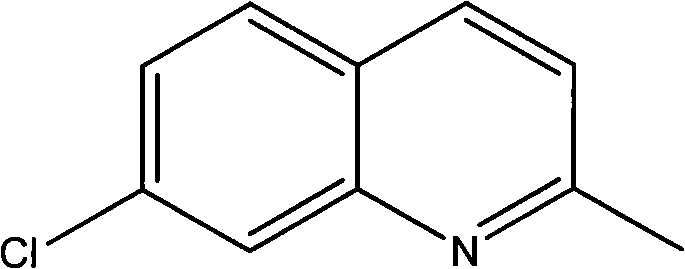
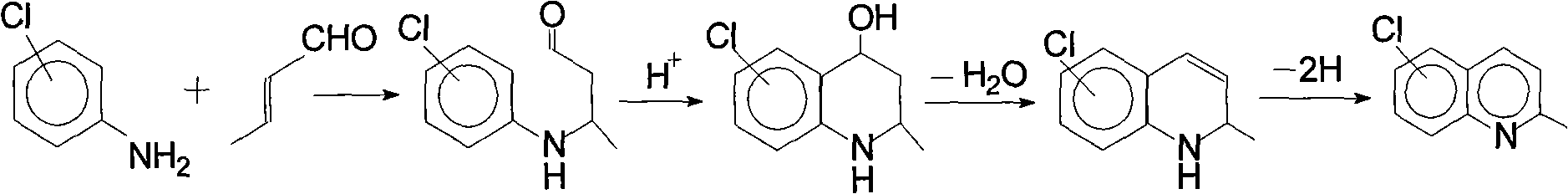
Method for preparing 7-chloroquinaldine by utilizing phase-transfer reaction
A technology of phase transfer and phase transfer catalysts, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve problems such as low reaction yield and difficult impurity purification, and achieve reduction Polymerization side reaction, the effect of mild reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Step 1, m-chloroaniline is dissolved in organic solvent
[0041] Dissolve 192 g (1500 mmol) of m-chloroaniline in 250 ml of 2-ethylhexanol and 150 ml of toluene, and stir evenly.
[0042] Step 2, synthesis reaction
[0043] A 2000 ml four-necked glass flask equipped with mechanical stirring, a thermometer, a spherical reflux condenser, and a dropping funnel, successively added 600 ml of 31% mass fraction (5800 mmol) of industrial grade hydrochloric acid and 10 g of alkylphenol polyoxyethylene ether to the flask. OP-10, 2.5 grams of benzyltriethylamine chloride, 185 grams (750 mmol) of tetrachloro-p-benzoquinone, 12 grams (72 mmol) of potassium iodide, 8.2 grams (32 mmol) of iodine, 1.2 grams (10 mmol) of p-dimethylaminopyridine ( DMAP). Then add the already prepared m-chloroaniline solution dropwise into the flask through the dropping funnel under stirring at room temperature within 15 minutes. Due to the exotherm of the neutralization reaction, the temperature of th...
Embodiment 2
[0047] Step 1, m-chloroaniline is dissolved in organic solvent
[0048] Dissolve 192 g (1500 mmol) m-chloroaniline in 250 ml C 4 ~C 9 Mix low-carbon alcohol and 150 ml of sec-butanol, and stir evenly.
[0049] Step 2, synthesis reaction
[0050] A 2000 ml four-necked glass flask equipped with mechanical stirring, a thermometer, a spherical reflux condenser, and a dropping funnel, successively added 600 ml of 31% mass fraction (5800 mmol) of industrial grade hydrochloric acid and 10 g of alkylphenol polyoxyethylene ether to the flask. OP-10, 86 grams (627 mmol) o-nitrotoluene, 1.2 grams (10 mmol) p-dimethylaminopyridine (DMAP). Then add the already prepared m-chloroaniline solution dropwise into the flask through the dropping funnel under stirring at room temperature within 15 minutes. Due to the exotherm of the neutralization reaction, the temperature of the reaction solution naturally rose to 50-60°C, and white m-chloroaniline hydrochloride was precipitated. The reaction...
Embodiment 3
[0054] Step 1, m-chloroaniline is dissolved in organic solvent
[0055] Dissolve 192 grams (1500 mmol) of m-chloroaniline in 250 milliliters of secondary octanol and 150 milliliters of refined fusel oil, and stir evenly.
[0056] Step 2, synthesis reaction
[0057] A 2000 ml four-necked glass flask equipped with mechanical stirring, a thermometer, a spherical reflux condenser, and a dropping funnel, successively added 600 ml of 31% mass fraction (5800 mmol) of industrial grade hydrochloric acid and 10 g of alkylphenol polyoxyethylene ether to the flask. OP-10, 100 grams (635 mmol) m-nitrochlorobenzene, 12 grams (72 mmol) potassium iodide, 8.2 grams (32 mmol) iodine. Then add the already prepared m-chloroaniline solution dropwise into the flask through the dropping funnel under stirring at room temperature within 15 minutes. Due to the exotherm of the neutralization reaction, the temperature of the reaction solution naturally rose to 50-60°C, and white m-chloroaniline hydroch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com