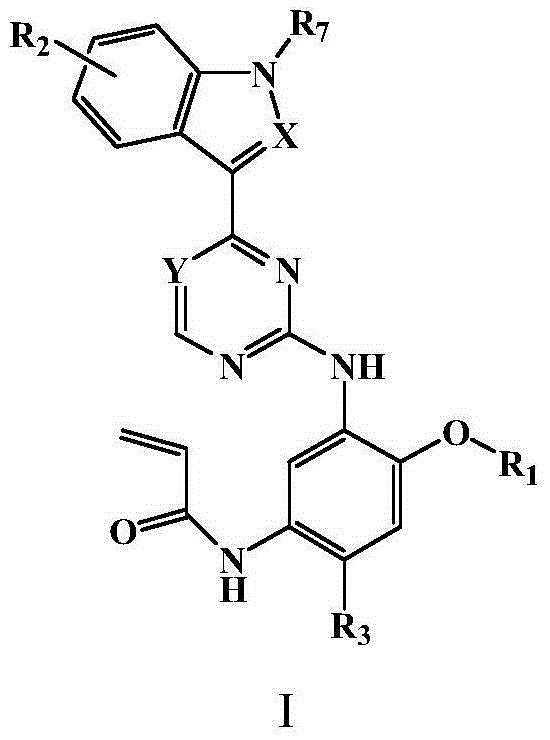
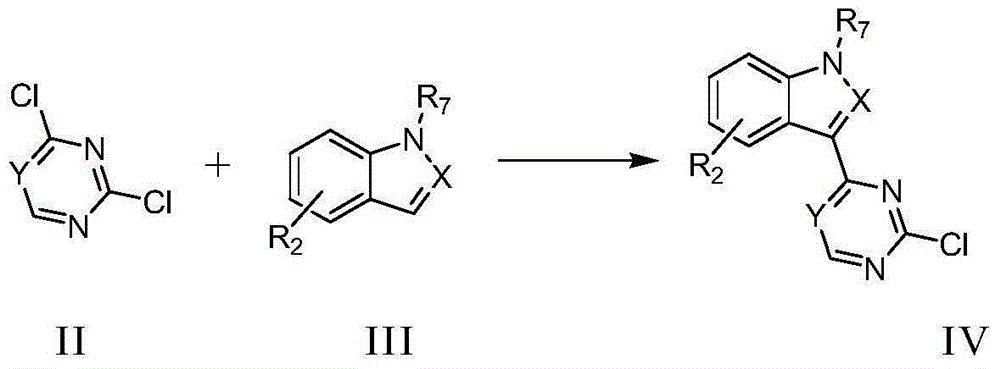
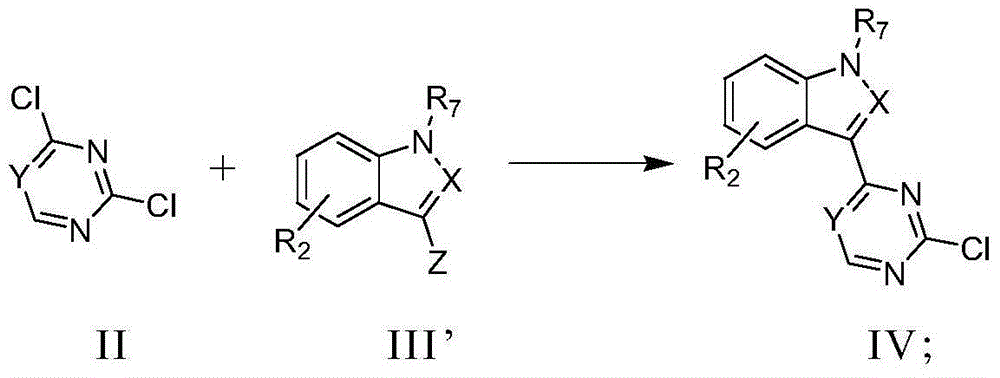
Pyrimidine or triazine derivative, and preparation method and use thereof
A pyrimidine and compound technology, applied in the fields of pyrimidine or triazine derivatives and their preparation and use, can solve the problems of drug-resistant tumors showing no obvious curative effect, limiting clinical drug dosage, and failing to reach the effective exposure of drug-resistant tumors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Example 1: 2-((2-acrylamido-5-methoxy-4-(4-(1-methyl-1H-indol-3-yl)-5-(trifluoromethyl) Synthesis of tert-butyl pyrimidin-2-ylamino)phenyl)(methyl)amino)ethyl(methyl)carbamate (compound 1):
[0134]
[0135] a. Synthesis of 3-(2-chloro-5-(trifluoromethyl)pyrimidin-4-yl)-1-methyl-1H-indole
[0136]
[0137] 5-(trifluoromethyl)pyrimidine-2,2-(1H,3H)-dione (18g, 0.1mol) was added to a 250ml three-necked flask, phosphorus oxychloride (45.8ml, 5eq) was added, Add phosphoric acid (0.1eq) under the protection of nitrogen, heat in an oil bath, slowly add diisopropylethylamine (16ml) dropwise between 85°C and 90°C, heat up to 100°C after dropping, reflux for 36h, evaporate to dryness under reduced pressure solvent, and column chromatography to obtain oily liquid 2,4-dichloro-5-(trifluoromethyl)pyrimidine (3.8 g, yield 17.7%).
[0138] Add 2,4-dichloro-5-(trifluoromethyl)pyrimidine (2.16g, 10mmol) into a 150ml single-necked bottle, add 30ml of anhydrous ethylene glycol ...
Embodiment 2
[0148] Example 2: N-(4-methoxy-2-(methyl(2-(methylamino)ethyl)amino)-5-(4-(1-methyl-1H-indole-3 - yl)-5-(trifluoromethyl)pyrimidin-2-ylamino)phenyl)acrylamide (compound 2):
[0149]
[0150] Compound 1 (354 mg) obtained in Example 1 was dissolved in 5 ml of dichloromethane, 3 ml of trifluoroacetic acid was added, stirred at room temperature for 2 h, TLC detected that the reaction of the raw materials was complete, the temperature was lowered to below 0 ° C, and 50 ml of saturated sodium bicarbonate and 50 ml of dichloromethane were added. Chloromethane, stirred for 30 min, separated, the aqueous phase was extracted three times with an equal amount of dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the target product (compound 2) (246 mg, yield 82%).
[0151] 1 H-NMR (400MHz, DMSO-d6, δppm): 9.48(s,1H), 9.10(s,1H), 8.64(s,3H), 8.25(s,1H), 8.13(m,1H), 7.88( s,1H), 7.48(d,1H, J=7.2H...
Embodiment 3
[0154] Example 3: 2-((2-acrylamido-5-ethoxy-4-(4-(1-methyl-1H-indol-3-yl)-pyrimidin-2-ylamino) Synthesis of phenyl) (methyl) amino) ethyl (methyl) tert-butyl carbamate (compound 3):
[0155]
[0156] a. Synthesis of 2-ethoxy-4-fluoro-5-nitroaniline:
[0157]
[0158] Suspend 5-fluoro-2-nitrophenol (6g, 1eq), potassium carbonate (15.8g, 3eq) in 150ml N,N-dimethylformamide, drop bromoethane (8.3g, 2eq), The temperature was raised to 37° C. and the reaction was stirred. After the reaction was detected by TLC, the system was poured into ice water, and the product was precipitated, filtered and washed with water to obtain 5-fluoro-2-nitrophenetole (6.8 g, yield 97%).
[0159] Dissolve 5-fluoro-2-nitrophenetole (6.8g) in 150ml of methanol, add 10% Pd / C (680mg), replace with hydrogen, stir and add hydrogen at room temperature, after the reaction is detected by TLC, filter, and the filtrate The solvent was distilled off under pressure to obtain 4-fluoro-2-ethoxyaniline (5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com