2 - (2, 4, 5 - substituted -anilino) pyrimidine derivatives as egfr modulators useful for treating cancer
A pyrimidine and 5-a technology, applied in the field of 2-pyrimidine compounds and their pharmaceutically acceptable salts, can solve the problems of increased affinity and decreased affinity of adenosine triphosphate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
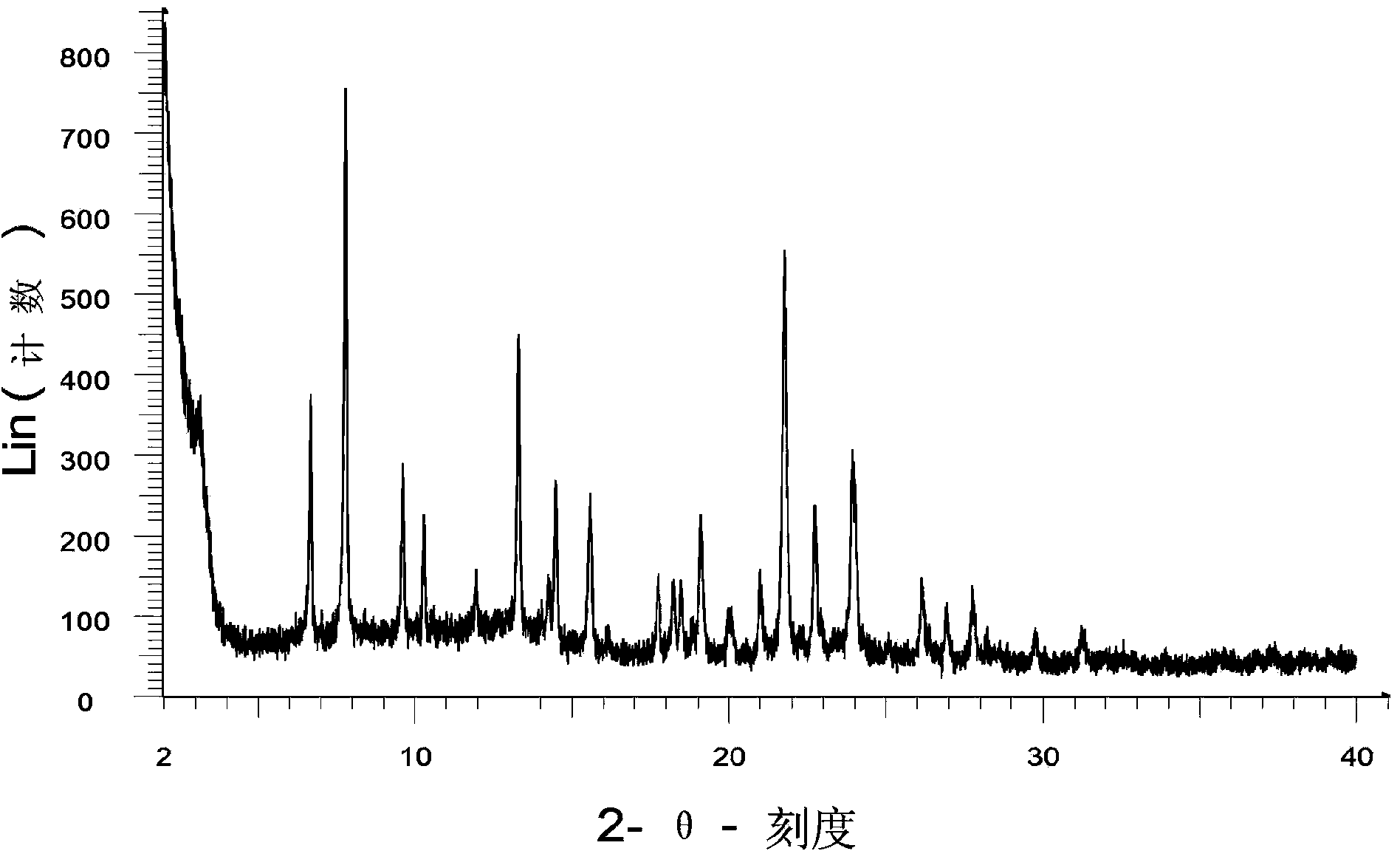
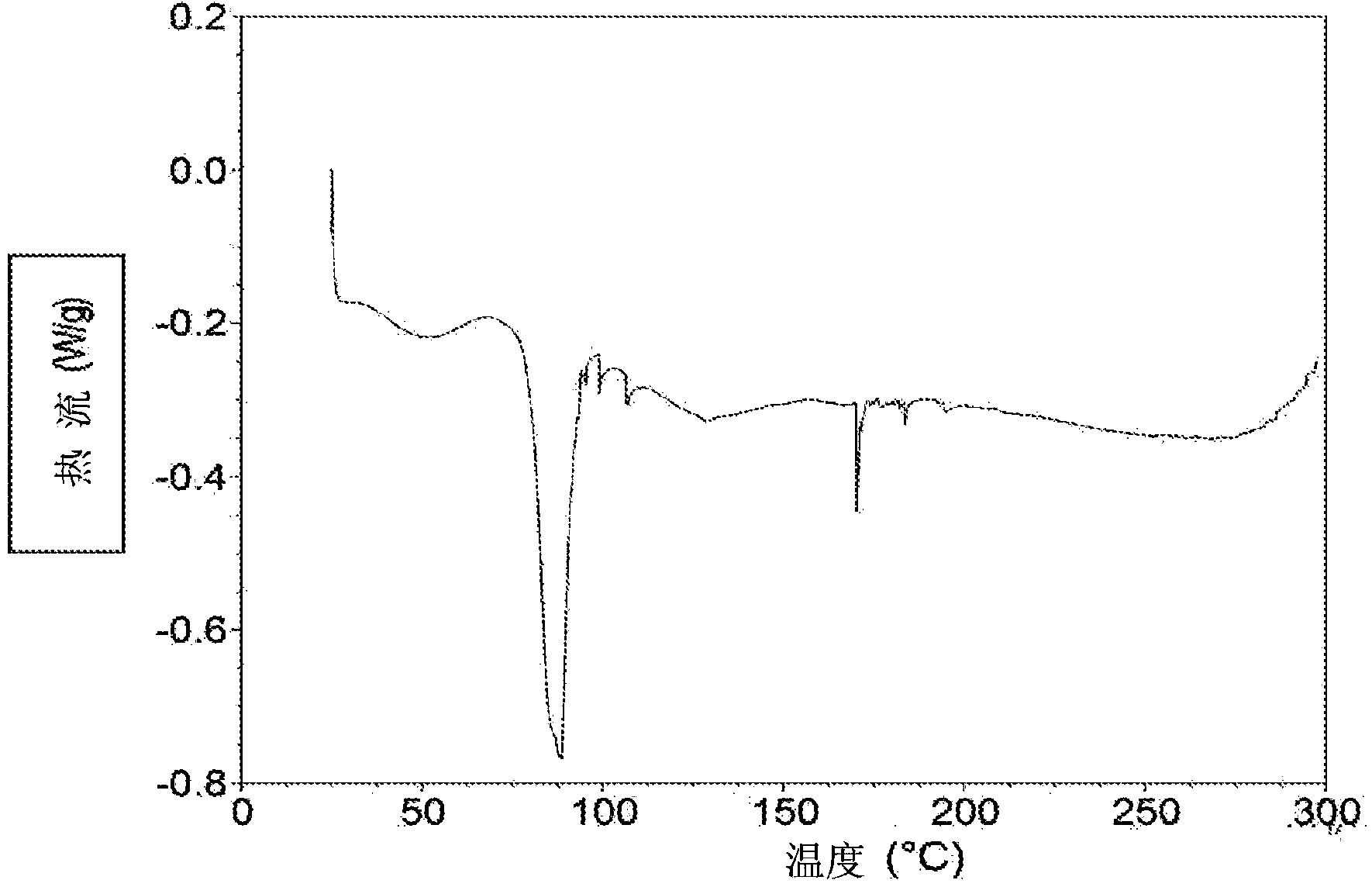
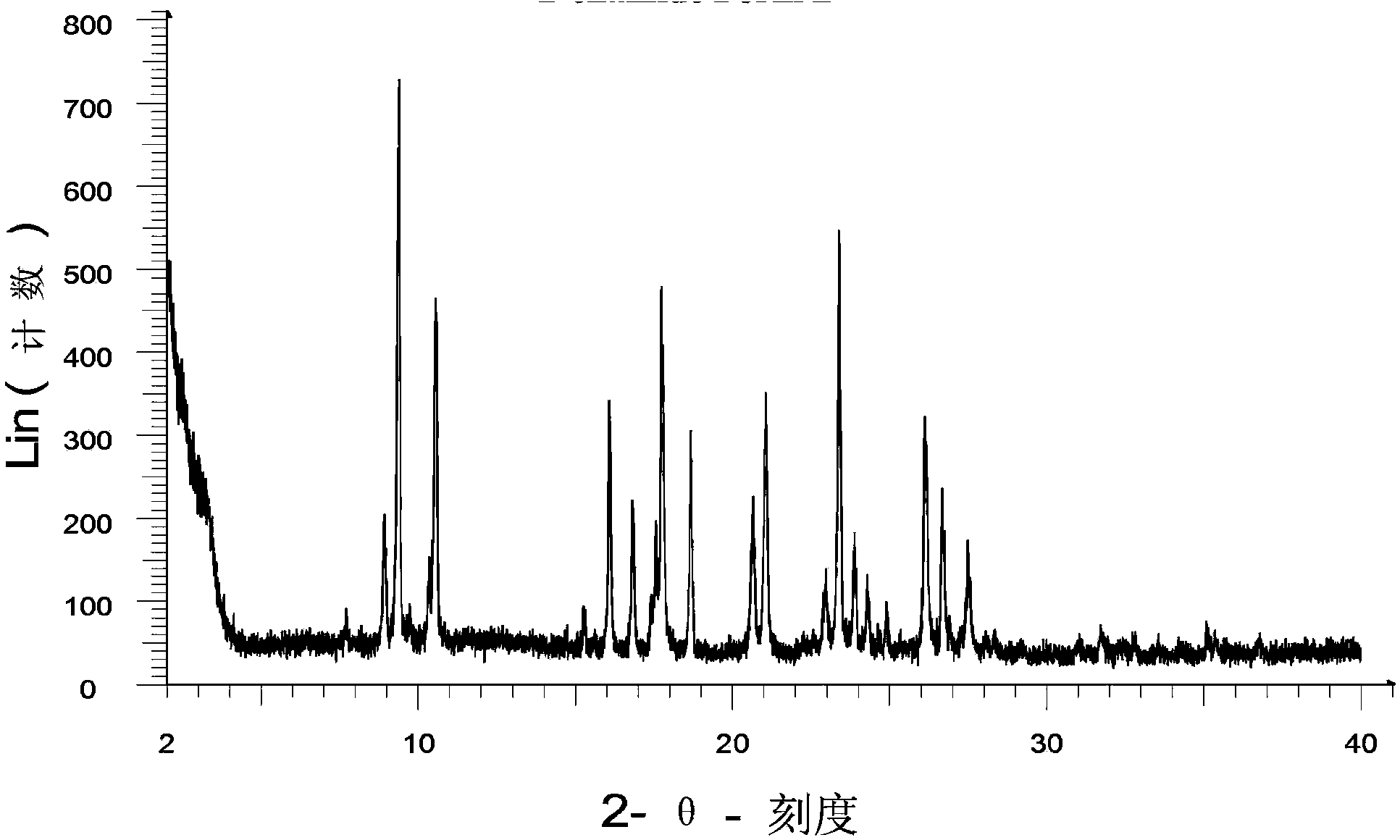
Image
Examples
Embodiment 1
[0495] Example 1: N-{4-methoxy-2-[1-methyl-3,6-dihydro-2H-pyridin-4-yl]-5-[(5-methyl-4-pyrazole A[1,5-a]pyridin-3-ylpyrimidin-2-yl)amino]phenyl}prop-2-enamide
[0496] at -10°C in N 2 Acryloyl chloride (0.331 mL, 1 M in THF, 0.33 mmol) was added dropwise to 6-methoxy-4-(1-methyl-1,2,3,6-tetrahydropyridine- 4-yl)-N1-[5-methyl-4-(pyrazolo[1,5-a]-pyridin-3-yl)pyrimidin-2-yl]benzene-1,3-diamine (intermediate 1, 146 mg, 0.33 mmol) and DIPEA (0.086 mL, 0.50 mmol) in THF (4 mL). The resulting mixture was stirred at 0 °C for 15 minutes, then concentrated in vacuo. Dissolve the residue in CH 2 Cl 2 (5mL) plus a small amount of CH 3 OH. Then, with saturated NaHCO 3 (2 mL) wash the solution, dry (MgSO 4 ), and then concentrated in vacuo. Purified by FCC with CH 2 Cl 2 5-25% CH in 3 OH, the appropriate fractions were concentrated in vacuo, and the obtained material was dissolved in CH 2 Cl 2 :7N methanol-ammonia 100:8 (1 mL), filter through a 1 g plug of silica gel. Conc...
Embodiment 2
[0497] Example 2: N-(5-{[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino}-4-methoxy-2-[1-methyl- 3,6-Dihydro-2H-pyridin-4-yl]phenyl)prop-2-enamide
[0498] at -5°C in N 2 Acryloyl chloride (0.217 mL, 1 M in THF, 0.22 mmol) was added dropwise to N'-[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl] in 1 min -4-Methoxy-6-(1-methyl-3,6-dihydro-2H-pyridin-4-yl)benzene-1,3-diamine (Intermediate 7, 100 mg, 0.22 mmol) and DIPEA (0.057 mL, 0.33 mmol) in THF (3 mL). The resulting mixture was stirred at 0 °C for 15 minutes, then concentrated in vacuo. Dissolve the residue in CH 2 Cl 2 (5mL) add a few drops of CH 3 OH, with saturated NaHCO 3 solution (2 mL) for washing. Then, the organic solution was dried (MgSO 4 ), loaded onto silica gel under vacuum. Purified by FCC, used in CH 2 Cl 2 5-25% CH in 3 OH, and the appropriate fractions were concentrated in vacuo to obtain a residue, which was washed with CH 3 OH (0.3 mL) and dried in air. The title compound (37 mg, 31%) was obtain...
Embodiment 3
[0500] Example 3: N-(5-{[4-(1H-indol-3-yl)-5-methylpyrimidin-2-yl]amino}-4-methoxy-2-[4-methyl piperazin-1-yl]phenyl)prop-2-enamide
[0501] at 0°C in N 2 , acryloyl chloride (0.025 mL, 0.30 mmol) was added dropwise to N'-[4-(1H-indol-3-yl)-5-methylpyrimidin-2-yl]-4-methoxy- 6-(4-Methylpiperazin-1-yl)benzene-1,3-diamine (Intermediate 12, 135 mg, 0.30 mmol) and DIPEA (0.090 mL, 0.33 mmol) in CH 2 Cl 2 (10 mL) and DMF (2 mL). The resulting suspension was stirred at 0°C for 2 hours. Then warm to room temperature. The mixture was then diluted with water (15 mL) and washed with CH 2 Cl 2 (40 mL) for extraction. The resulting organic solution was washed with saturated Na 2 CO 3 (20 mL) and washed with saturated brine (20 mL). The solution was then dried (MgSO 4 ), concentrated in vacuo. Purified by FCC, used in CH 2 Cl 2 Elution with 1-5% 7M Methanol-Ammonia in Ethanol afforded the crude product. Further purification was carried out by preparative HPLC (Waters SunF...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com