Overcoming resistance to ERBB pathway inhibitors
A technology of inhibitors and small molecule inhibitors, applied in the direction of antibodies, anti-tumor drugs, antibody medical components, etc., can solve problems such as lack of phosphorylation and AKT phosphorylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
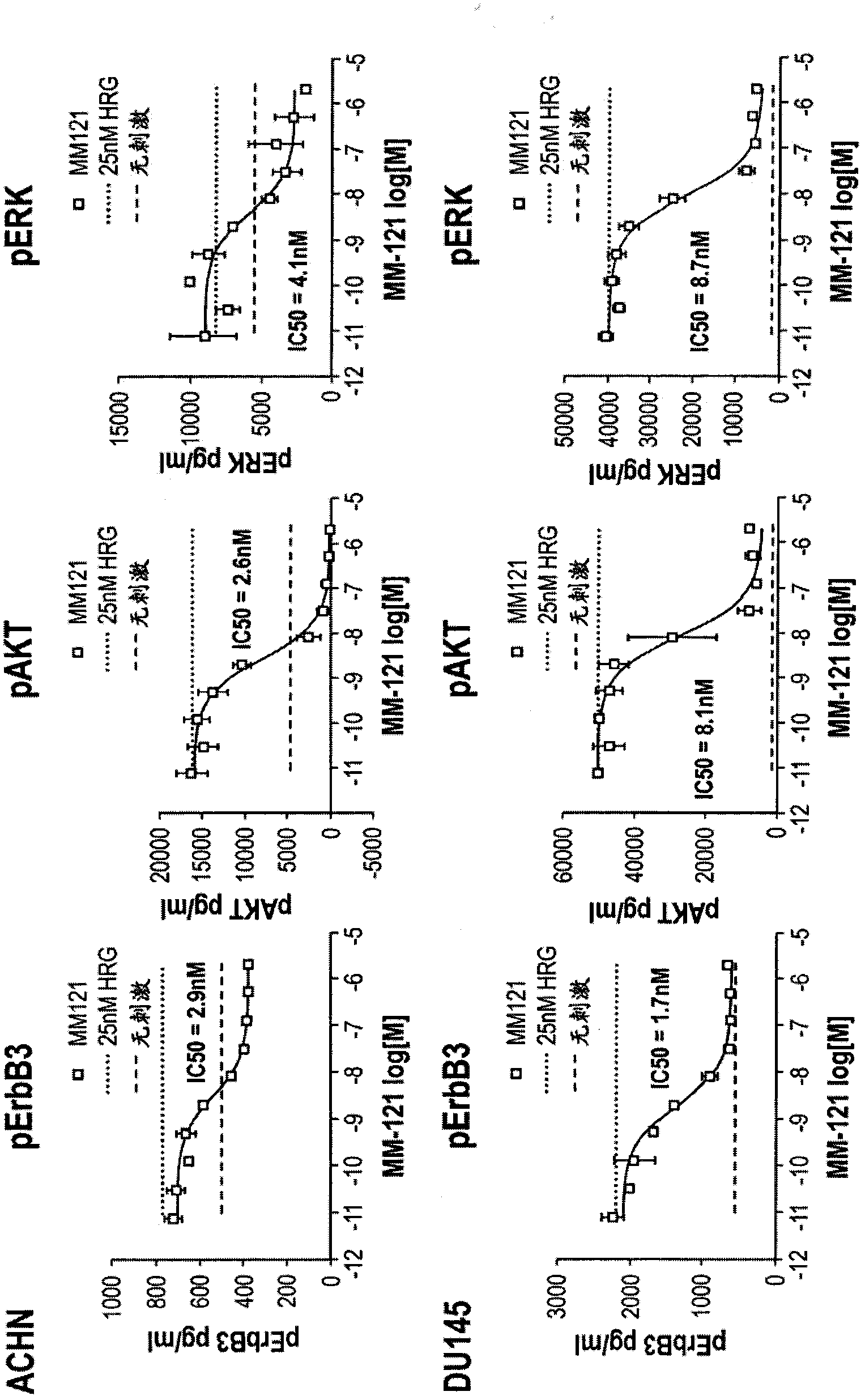
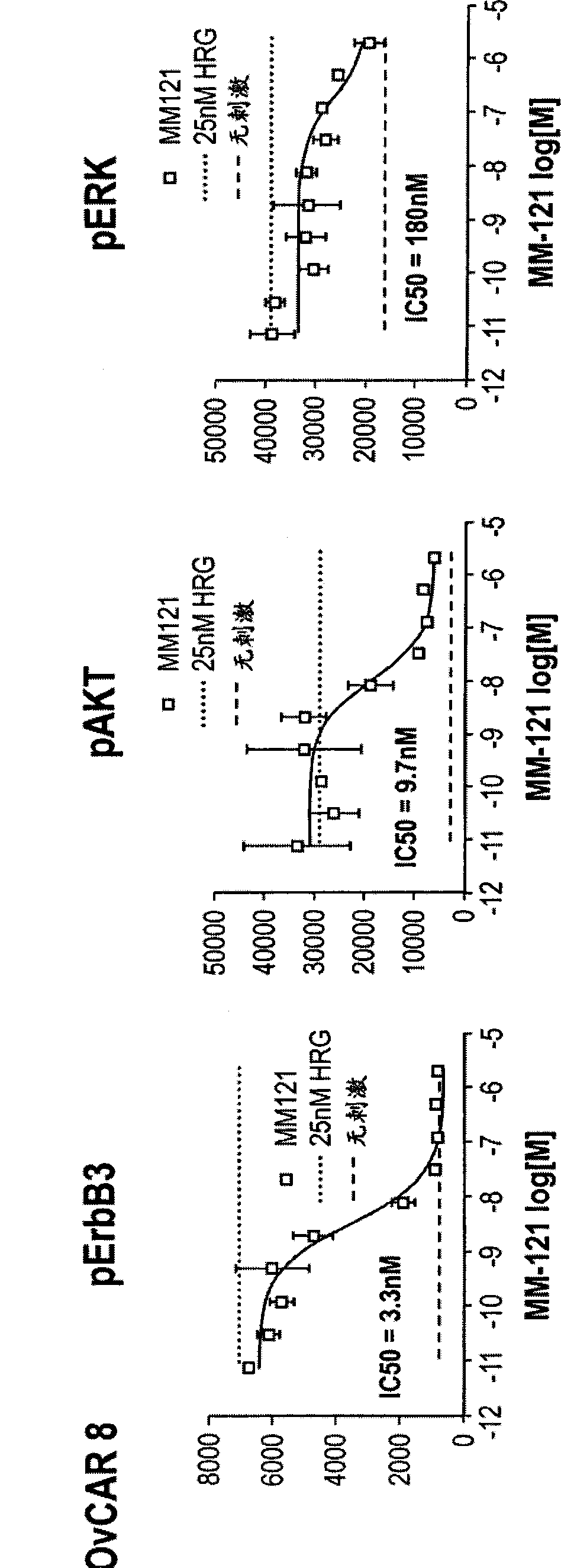
[0120] Example 1: MM-121 blocks ligand-induced ErbB3 activation
[0121] In this example, the anti-ErbB3 monoclonal antibody MM-121 (Ab#6, as disclosed in U.S. Patent No. 7,846,440) was examined in a series of in vitro experiments for its inhibition of ligand-induced activation of ErbB3 phosphorylation and Ability to transduce signals.
[0122] In vitro signaling studies measured by ELISA
[0123] In the first set of experiments, 3 cancer cell lines (ACHN (renal cell adenocarcinoma, ATCC #CRL-1611 TM ), Du145 (prostate cancer, ATCC #HTB-81 TM ) and OvCAR8 (ovarian adenocarcinoma, ATCC #HTB-161 TM) cell line; obtained from the Developmental Therapeutics Program (Developmental Therapeutics Program) of the National Cancer Institute (National Cancer Institute)) were seeded into 96-well plates and cultured overnight (maintenance medium was supplemented with 10% fetal calf serum , 2mM L-glutamine, and Pen-Strep in RPMI-1640 medium; in a humidity-controlled atmosphere at 5%...
Embodiment 2
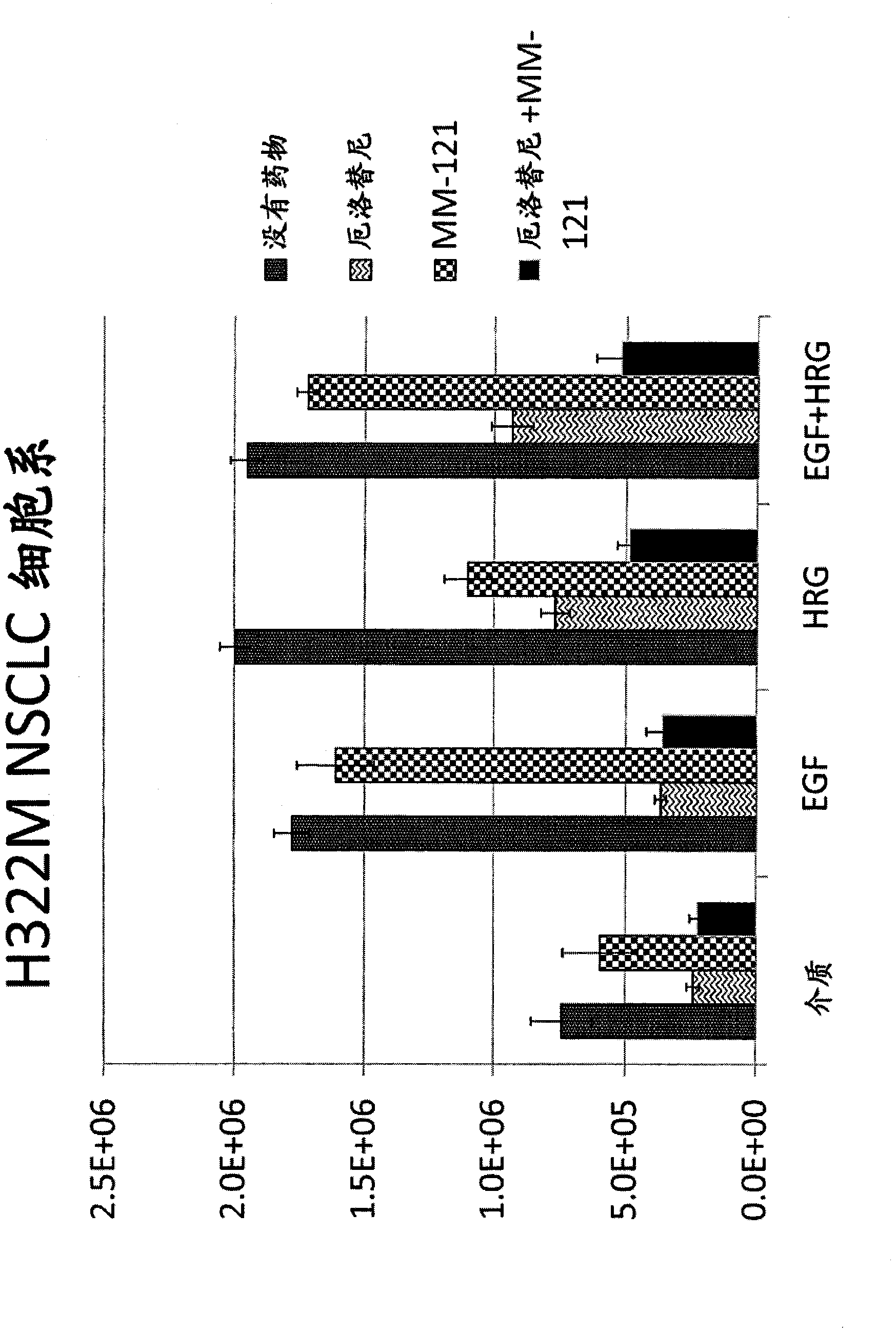
[0126] Example 2: MM-121 overcomes resistance to erlotinib in an in vitro model of EGFR-wild-type non-small cell lung cancer
[0127] method
[0128] Nine non-small cell lung cancer (NSCLC) cell lines were obtained from the American Type Culture Collection: A549 (ATCC #CCL-185 TM ), EKVX (NCI vial0502454), NCI-H2170 (ATCC #CRL-5928 TM ), NCI-H2347 (ATCC #CRL-5942 TM ), NCI-H322M (ATCC #CRL-5806 TM ), NCI-H358 (ATCC #CRL-5806 TM ), NCI-H441 (ATCC #HTB-174 TM ), NCI-H661 (ATCC #HTB-183 TM ) and SW-900 (ATCC #HTB-59 TM ). The cell lines carry no mutations in their EGFR gene and represent 3 distinct histological subtypes (see Table X below). Cells carrying Ras mutations are indicated.
[0129] At 5,000 cells / well, cells were seeded in a 96-well 3D-culture system (low-binding NanoCulture plate, ScivaX Corporation), and cultured at 37°C in RPMI-1640 medium supplemented with 10% fetal bovine serum and Pen-Strep. After 48 h (after which time spheroids had f...
Embodiment 3
[0139] Example 3: Inhibition of pAKT production in human ovarian cancer cells in vitro
[0140] Materials and methods:
[0141] The A2780 human ovarian carcinoma cell line was initially established from tumor tissue obtained from untreated patients. The A2780cis cell line is cisplatin resistant (Catalog #93112517, Sigma). It is formed by chronic exposure of the cisplatin-sensitive parental A2780 cell line (cat. no. 93112519, Sigma) to increasing concentrations of cisplatin. A2780cis is cross-resistant to melphalan, doxorubicin and irradiation. To maintain resistance, cisplatin was added to the medium every 2-3 passages after attachment.
[0142]Resistance to cisplatin was demonstrated by treating A2780 and A2780cis cells with serial dilutions of cisplatin (0.01-10 [mu]M) for 72 hours. Cell viability was measured using the Cell Titer Glo Assay (Cat# G7570, Promega) according to the manufacturer's instructions.
[0143] The effect of cisplatin on the AKT pathway was determi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com