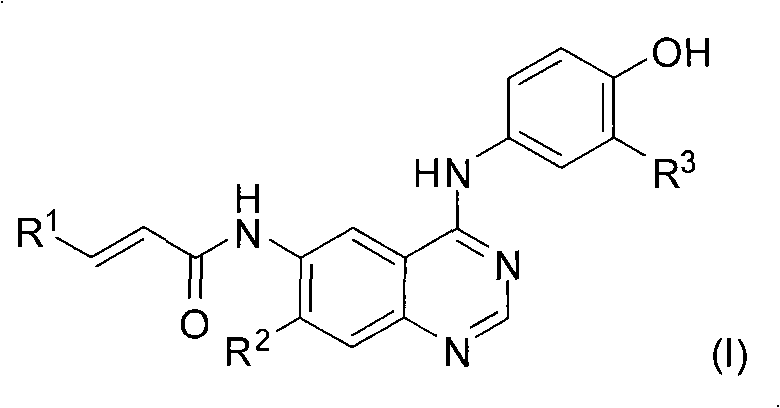
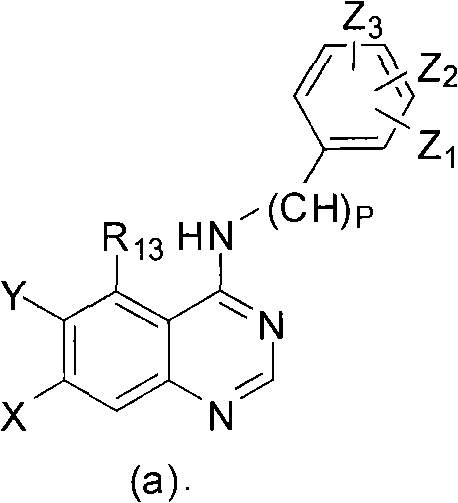
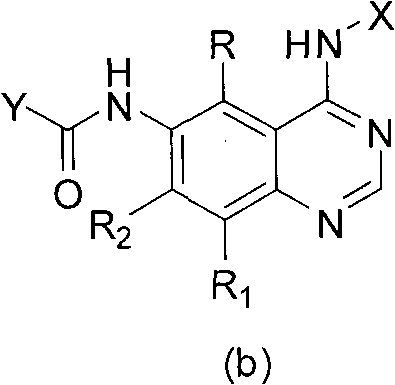
Quinazoline derivatives and preparation method and application thereof
A technology of quinazoline and derivatives, which is applied in the field of quinazoline derivatives and can solve problems such as side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] N-[4-(3-chloro-4-hydroxy-phenylamino)-7-methoxy-quinazolin-6-yl-]-crotonamide
[0050]
[0051] In a flask equipped with a condensing device, the raw material (Ia-1) 4-hydroxy-3-chloroaniline 1.37 g (5.6 mmol) and (Ib-1) 6-nitro-7-methoxy-4- 1.20g (5.7mmol) of chloro-quinazoline was dissolved in 80ml of isopropanol and reacted under reflux for 3h. A large amount of yellow solid precipitated in the system, filtered, and washed with saturated sodium bicarbonate aqueous solution to pH=8. The sample was dried in vacuum, and the compound was identified as (Ic-1).
[0052] Add 1.60g (3.77mmol) of the above (Ic-1) compound, 1.05g (18.85mmol, 5eq) of reduced iron powder, 2ml of glacial acetic acid, 40ml of methanol, and reflux under an oil bath at 85℃ into a flask equipped with reflux condenser After 2.5 hours of reaction, the iron powder was removed by filtration, the filtrate was diluted with ethyl acetate, washed with sodium bicarbonate solution, washed with water, and the organ...
Embodiment 2
[0058] N-[4-(3-Chloro-4-hydroxy-phenylamino)-quinazolin-6-yl-]-crotonamide
[0059]
[0060] In a flask equipped with a condenser, the raw materials (Ia-1) 4-hydroxy-3-chloroaniline 1.37 g (5.6 mmol) and (Ib-2) 6-nitro-4-chloro-quinazoline 1.20 g (5.7 mmol) was dissolved in 80ml of isopropanol and reacted under reflux for 3h. A large amount of yellow solid precipitated in the system, filtered, and the solid was washed with saturated sodium bicarbonate aqueous solution to pH=8. The sample was dried in vacuum, and the compound was identified as (Ic-2).
[0061] Add 1.60g (3.77mmol) of the above (Ic-2) compound, 1.05g (18.85mmol, 5eq) of reduced iron powder, 2ml of glacial acetic acid, 40ml of methanol, and reflux under an oil bath at 85°C into a flask equipped with a reflux condenser After 2.5 hours of reaction, the iron powder was removed by filtration, the filtrate was diluted with ethyl acetate, washed with sodium bicarbonate solution, washed with water, and the organic phase was...
Embodiment 3
[0067] N-[4-(3-Chloro-4-hydroxy-phenylamino)-quinazolin-6-yl-]-acrylamide
[0068]
[0069] In a 100ml flask, add 1.2g (3.04mmol) of the (Id-2) compound of Example 2 above, 0.8ml (6.1mmol, 2eq) of triethylamine, 1.02ml (12.1mmol, 4eq) of acryloyl chloride under ice bath ), THF40ml, gradually warmed to room temperature for reaction. After 3h, the reaction was stopped, filtered, the solid was washed with water to neutrality, and dried to obtain 1.1g of solid. The compound was identified as (3) with a yield of 68%.
[0070] H 1 -NMR (400MHz, CDCl 3 +DMSO):
[0071] δ8.63(s, 1H), 8.53(s, 1H), 8.14(s, 3H), 7.71-7.64(dd, 2H), 7.23(s, 1H), 7.06-7.02(m, 2H), 6.78- 6.76(d, 1H), 5.92-5.89(d, 1H), 5.54-5.48(m, 1H)
[0072] ESI(+): 341
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com