Albumin nanometer particle preparation for soluble injection and preparation method of albumin nanometer particle preparation
A technology for albumin nanoparticles and injections, applied in the field of pharmaceutical preparations, can solve the problems of frequent administration, low oral bioavailability, and increased toxic and side effects, so as to avoid low bioavailability and reduce systemic toxicity Side effects, effects of avoiding gastrointestinal toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
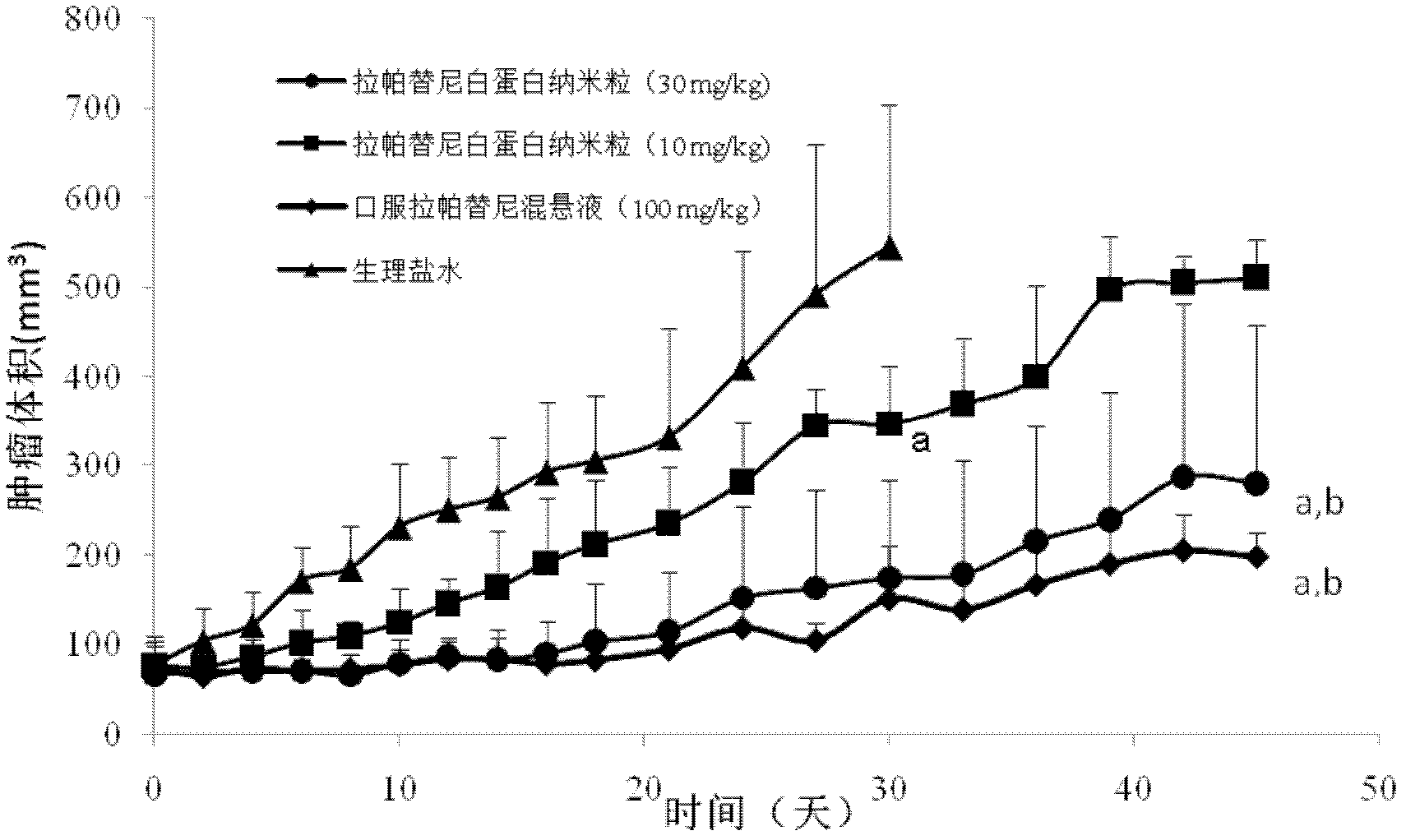
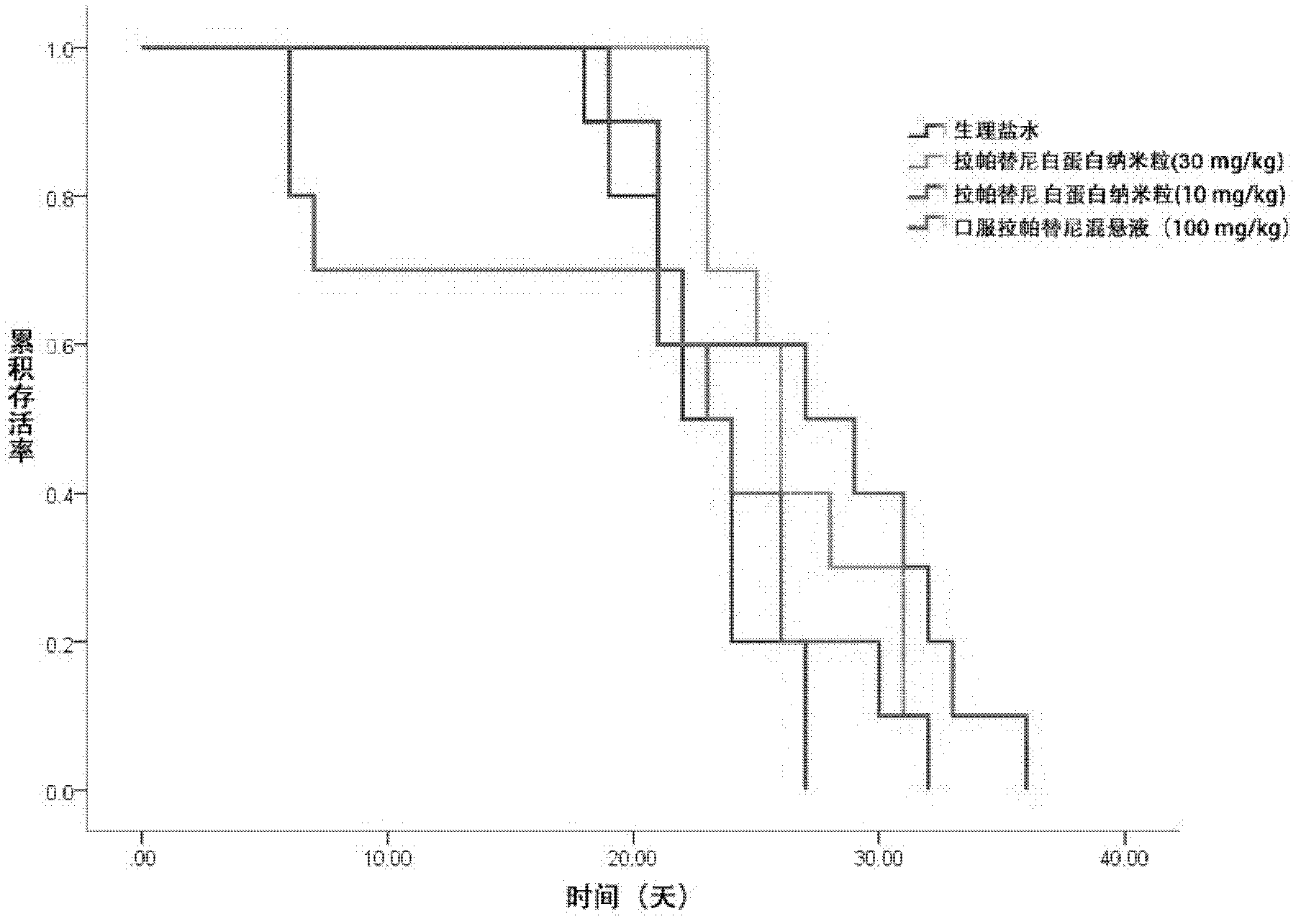
[0040] Example 1. Prescription process of lapatinib albumin nanoparticles
[0041] Table 1 The proportion of each component in the prescription of albumin nanoparticles containing lapatinib (mg)
[0042]
[0043] Respectively dissolve the lapatinib in the prescriptions 1 to 30 in Table 1 in an appropriate amount of ethanol-water (6:1 to 2:1) mixed solvent or other appropriate solvent, dissolve the phospholipid in an appropriate amount of dichloromethane, and dissolve the two Mix it as the oil phase; dissolve albumin and antioxidants in an appropriate amount of deionized water (water phase), drop the oil phase into the magnetically stirred water phase at room temperature or in an ice-water bath, and continue to stir for a certain period of time, then 40°C The ethanol and dichloromethane are removed by rotary evaporation in a water bath to obtain lapatinib-loaded albumin nanoparticles.
Embodiment 2
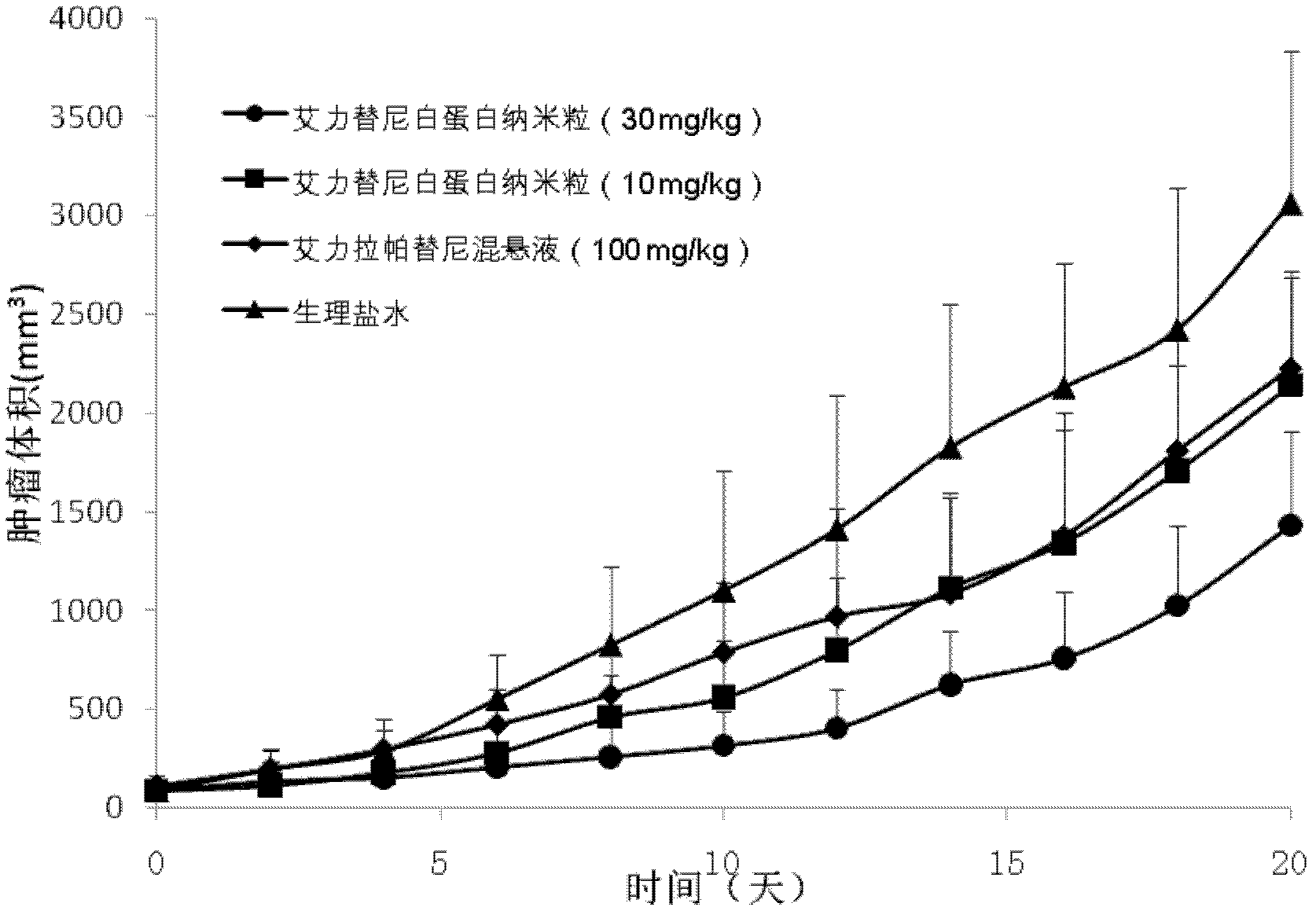
[0044] Example 2. Prescription technology of Ariitinib albumin nanoparticles
[0045] Table 2 Proportion (mg) of each component in the prescription of albumin nanoparticles containing eritinib
[0046]
[0047] Dissolve the eritinib in the prescriptions 1-30 of Table 2 in a suitable amount of ethanol-water (6:1~2:1) mixed solvent or other suitable solvents, dissolve the phospholipids in a suitable amount of dichloromethane, and mix the two Then it is used as the oil phase; dissolve albumin and antioxidants in an appropriate amount of deionized water (water phase), drop the oil phase into the magnetically stirred water phase at room temperature or in an ice water bath, and continue to stir for a certain period of time, then water bath at 40°C Rotary evaporation to remove ethanol and dichloromethane to obtain albumin nanoparticles loaded with eritinib.
Embodiment 3
[0048] Example 3. Prescription technology of gefitinib albumin nanoparticles
[0049] Table 3 Proportion (mg) of each component in the prescription of albumin nanoparticles containing gefitinib
[0050]
[0051] Dissolve gefitinib in prescriptions 1-30 in Table 3 in a suitable amount of ethanol-water (6:1~2:1) mixed solvent or other suitable solvents, dissolve the phospholipids in a suitable amount of dichloromethane, and mix the two Then it is used as the oil phase; the albumin and antioxidants are dissolved in an appropriate amount of deionized water (water phase), the oil phase is dropped into the magnetically stirred water phase at room temperature or in an ice water bath, and stirring is continued for a certain period of time. Then, the ethanol and dichloromethane were removed by rotary evaporation in a water bath at 40°C to obtain albumin nanoparticles carrying gefitinib.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com