Erlotinib-Cy7-chitosan polymer with tumor targeting
A chitosan polymer and tumor-targeting technology, applied in the field of biomedicine, can solve the problem of incompatibility, achieve the effects of overcoming poor solubility, improving therapeutic effect, and reducing drug dosage and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of Erlotinib-Cy7-Chitosan Polymer (CE7):
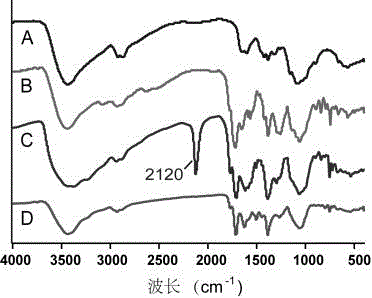
[0040] Step a: Weigh 200 mg of chitosan Cs (chitosan was purchased from Shanghai Boao Biotechnology Co., Ltd., with a molecular weight of 60 kilodaltons and a deacetylation degree of 90%) and dissolve it in 20 mL of anhydrous DMF, and then Add 800 mg of 4-bromophthalic anhydride, nitrogen protection, and heat with stirring in an oil bath at 125°C. When the reaction solution became clear and the solution was yellow, the reaction was terminated. Filter while hot, then directly pour the hot filtrate into 200mL ice water, and a white solid is precipitated. Suction filtration, the solid was washed three times with ether and acetone respectively to remove excess 4-bromophthalic anhydride, and dried in a ventilated place to obtain product 2 (Cs-Br).
[0041] Step b: Weigh 60 mg of product 2, add 6 mL of N-methylpyrrolidone (NMP), heat and stir to dissolve completely, add 100 mg of sodium azide (NaN 3 ), under nitrogen pro...
Embodiment 2
[0044] Synthesis of Chitosan-Cy7 Polymer:
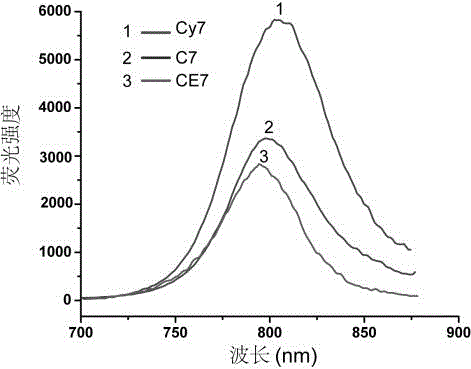
[0045] Weigh 30 mg of product 3, dissolve it in 3 mL of dimethyl sulfoxide, add to the flask, and then add 5 mg of Cy7. The flask was sealed with a rubber stopper. After vacuuming, under nitrogen protection, 4 mg of copper sulfate pentahydrate (dissolved in 200 μL of secondary water) was first added dropwise to the flask with a 1 mL syringe, and then 3 mg of sodium ascorbate (dissolved in 200 μL of secondary water) was added dropwise to the flask. secondary water). The reactants were reacted at 50 °C for 72 h in the dark. After the reaction, the reaction solution was dialyzed in secondary water for 72 hours with a 10,000 gauge dialysis bag. After dialysis, the product was lyophilized to yield polymer C7. C7 was dissolved in dimethyl sulfoxide, the excitation wavelength was 633nm, and the fluorescence intensity was measured. Such as figure 2 As shown, C7 has the characteristic peak of Cy7 at 790-810nm, indicating that Cy7 has be...
Embodiment 3
[0047] Synthesis of chitosan-erlotinib polymer:
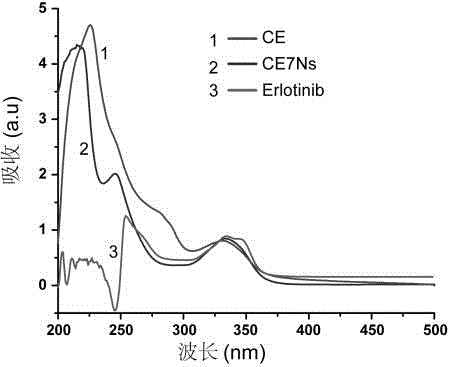
[0048] Weigh 30 mg of product 3, dissolve in 3 mL dimethyl sulfoxide, add to the flask, and then add 25 mg Erlotinib. The flask was sealed with a rubber stopper. After vacuuming, under nitrogen protection, 4 mg of copper sulfate pentahydrate (dissolved in 200 μL of secondary water) was first added dropwise to the flask with a 1 mL syringe, and then 3 mg of sodium ascorbate (dissolved in 200 μL of secondary water) was added dropwise to the flask. secondary water). The reactants were reacted at 50 °C for 72 h in the dark. After the reaction, the reaction solution was dialyzed in secondary water for 72 hours with a 10,000 gauge dialysis bag. After dialysis, the product was lyophilized to obtain polymer CE. The product was dissolved in dimethyl sulfoxide to measure the ultraviolet absorption, such as image 3 As shown, Erlotinib has a characteristic absorption peak at 330-350nm, and CE has ultraviolet absorption at 340nm, indic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com