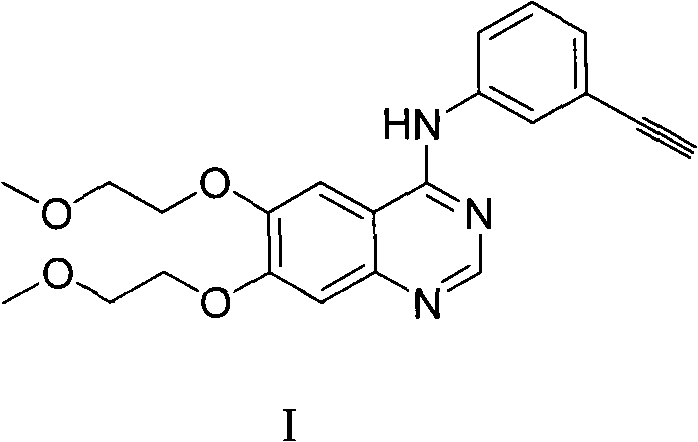
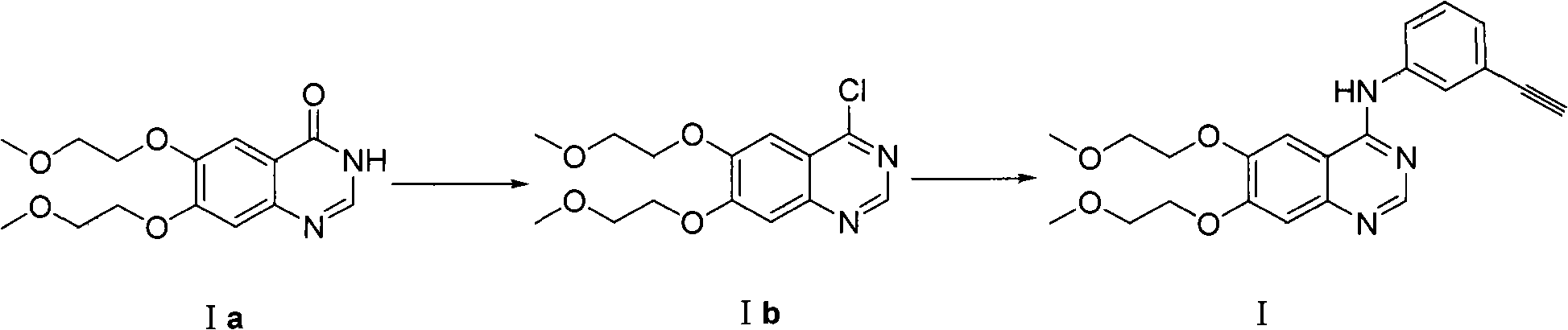
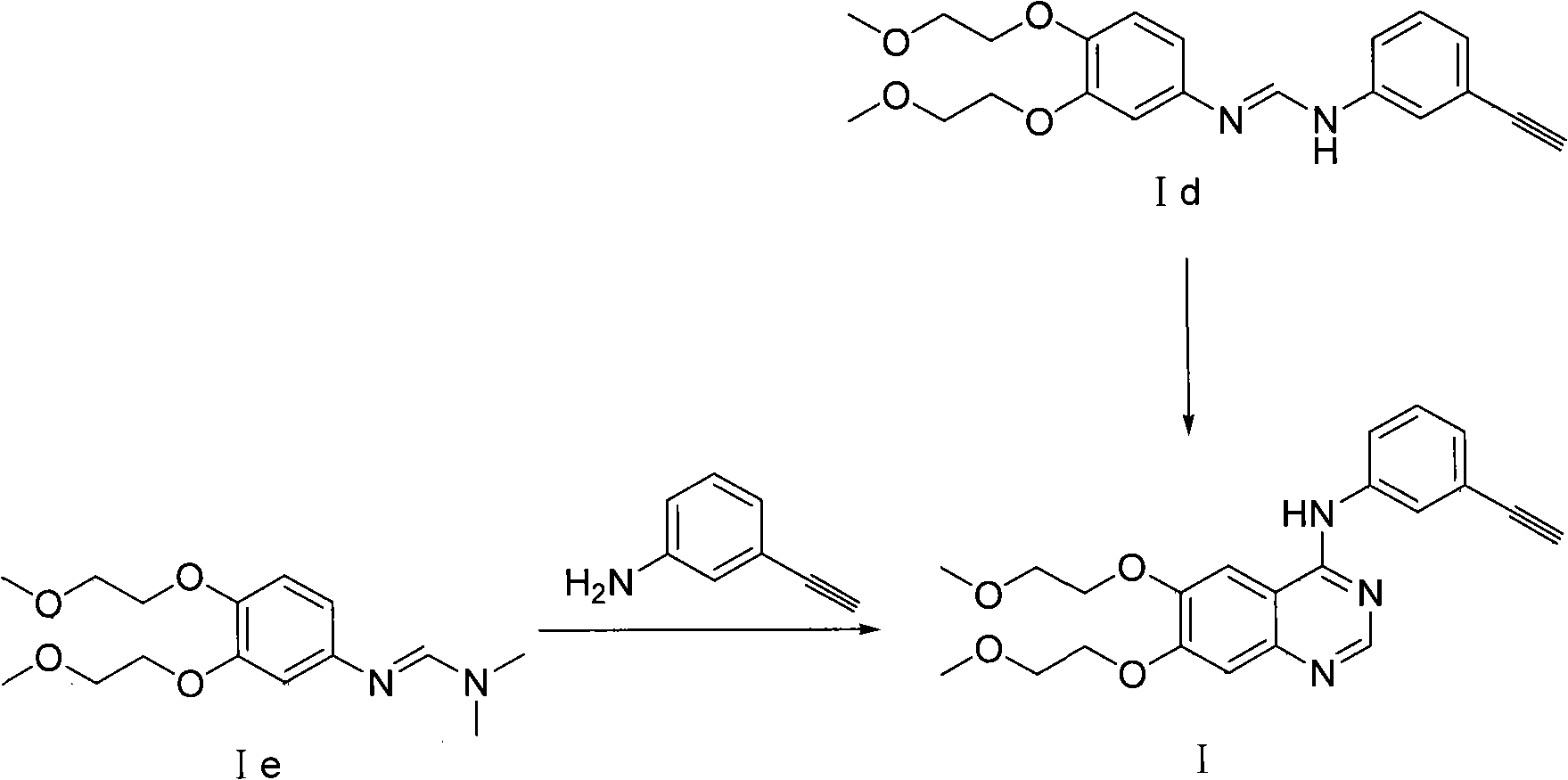
Preparation method of quinazoline derivate
A technology for quinazoline and derivatives, applied in the field of preparation of quinazoline derivatives, can solve the problems of large environmental pollution, low yield, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 compound I (compound I is R in the general formula compound I f 1 for H compounds)
[0049]
[0050] Under nitrogen protection, compound II (186mg, 0.63mmol) and benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (361mg, 0.83mmol) were dissolved in 6.3ml of acetonitrile, added 3-ethynylaniline (223mg, 1.9mmol) and 1,8-diazabicyclo-bicyclo(5,4,0)-7-undecene (0.15ml), stirring at room temperature for 12 hours, warming up to 60 °C, stirring was continued for 12 hours. The solvent was evaporated, and the residue was dissolved in 50ml of ethyl acetate, washed with aqueous sodium hydroxide solution (10ml, 1mol / l), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized from ethanol to obtain 125mg of compound I with a yield of 50 %.
[0051] Its structural identification data are as follows:
[0052] ESI-MS[M+H] + :295.1;
[0053] 1 H NMR (d 6 -DMSO): 11.44(s, 1H), 8.84(s, 1H),...
Embodiment 2
[0054] The preparation of embodiment 2 compound I
[0055]
[0056]Under nitrogen protection, compound II (186mg, 0.63mmol) and 2-(7-azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (0.63mmol) were dissolved 3-Ethynylaniline (0.63 mmol) and triethylamine (0.63 mmol) were added to 6.3 ml of dimethyl sulfoxide, and the reaction was stirred at -20°C. After the reaction was complete, the solvent was evaporated, and the residue was dissolved in 50 ml of ethyl acetate, washed with aqueous sodium hydroxide solution (10 ml, 1 mol / l), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized from ethanol to obtain 190 mg of compound I. The yield is 75%, and its structural identification data are the same as those in Example 1.
Embodiment 3
[0057] The preparation of embodiment 3 compound I
[0058]
[0059] Under nitrogen protection, compound II (186mg, 0.63mmol) and dicyclohexylcarbodiimide (3.2mmol) were dissolved in 6.3ml N,N-dimethylformamide, and 3-ethynylaniline (3.2mmol) and n-butyl Lithium (32mmol), stirred at 20°C. After the reaction was completed, the solvent was evaporated, and the residue was dissolved in 50 ml of ethyl acetate, washed with aqueous sodium hydroxide solution (10 ml, 1 mol / l), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized from ethanol to obtain 120 mg of compound I. The yield is 48%, and its structural identification data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com