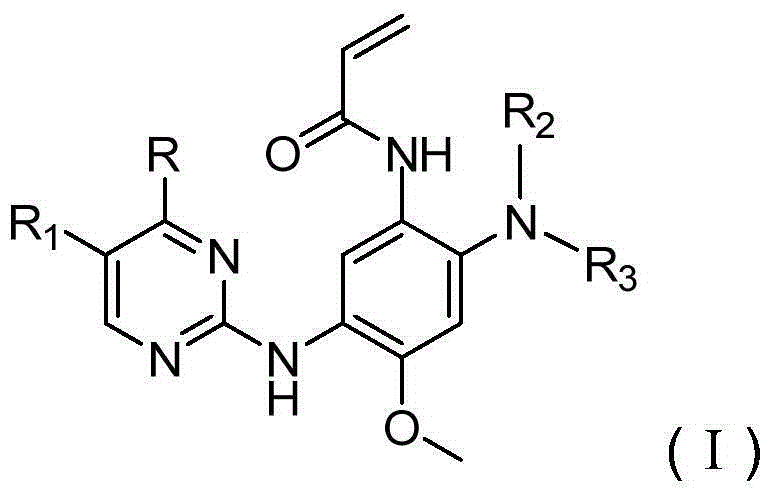
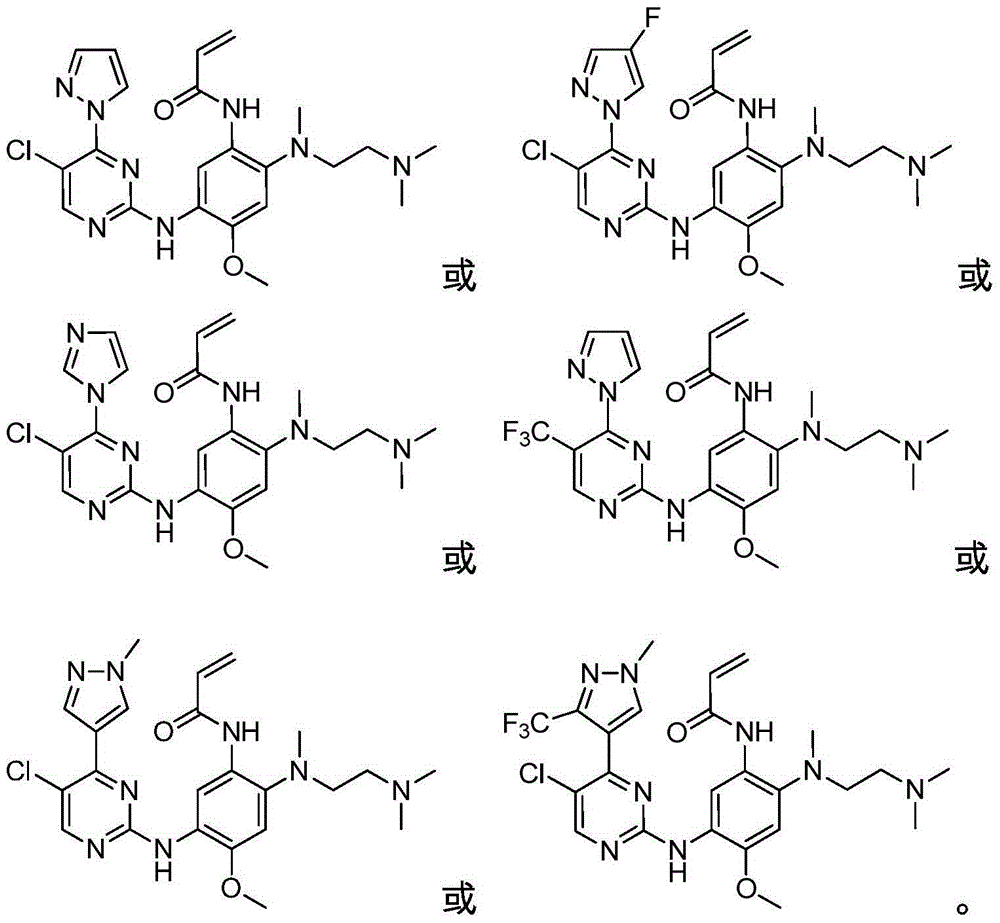
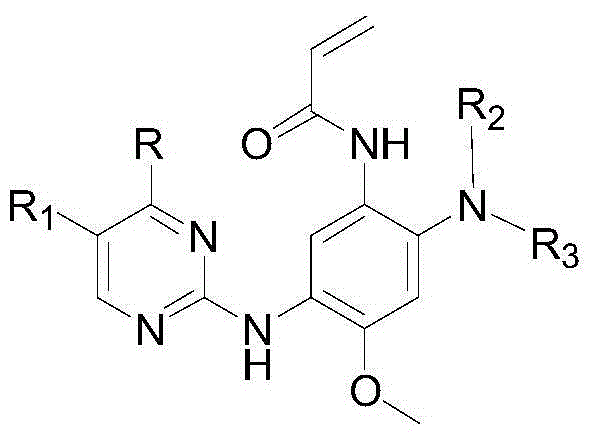
3-(2-pyrimidine amino) phenyl acrylic amide compound and application thereof
A compound and drug technology, applied to 3-phenylacrylamide compounds and their application fields in the preparation of antitumor drugs, can solve the loss of inhibitor activity, the side effects of wild-type cytotoxicity, the increase in the affinity of EGFR and ATP, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] N-(5-((5-Chloro-4-(1H-pyrazol-1-yl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl )amino)-4-methoxy)acrylamide
[0117]
[0118] The synthetic route is as follows:
[0119]
[0120] The concrete synthetic steps of above-mentioned synthetic route are as follows:
[0121] Step 1: Synthesis of 2,5-Chloro-4-(1H-pyrazol-1-yl)pyrimidine (Compound 3)
[0122] NaH (400 mg, 16.67 mmol) was suspended in dry tetrahydrofuran (30 mL), and pyrazole (340 mg, 5 mmol) was added at room temperature. The reaction was stirred at room temperature for 10 minutes and cooled to -30°C to -40°C. 2,4,5-Trichloropyrimidine (1.82 g, 10 mmol) was dissolved in tetrahydrofuran (5 mL) to make a solution, which was added at one time. The reaction was stirred at -30°C for 20 minutes, poured into ice water (50 g) and extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography...
Embodiment 2
[0133] N-(5-((5-Chloro-4-(4-fluoro1H-pyrazol-1-yl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl) (Methyl)amino)-4-methoxy)acrylamide
[0134]
[0135] The synthetic method refers to Example 1.
[0136] synthetic end product 1 H-NMR (400MHz, CDCl 3 ): δ9.53(s, 1H), 9.42(s, 1H), 8.49(s, 1H), 7.82(s, 1H), 7.73(d, J=4.2Hz, 1H), 6.73(s, 1H) ,6.56(d,J=1.4Hz,1H),5.75(d,J=11.7Hz,1H),3.90(s,3H),3.13(m,2H),2.88–2.40(m,10H).LC- MS: m / z 489[M+H] + .
Embodiment 3
[0138]N-(5-((5-Chloro-4-(1H-imidazol-1-yl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl) Amino)-4-methoxyphenyl)acrylamide
[0139]
[0140] The synthetic method refers to Example 1.
[0141] synthetic end product 1 H-NMR (400MHz, CDCl 3 )9.74(s,1H),δ9.70(brs,1H),8.92(brs,1H),8.8.24-8.26(m,2H),8.05(s,1H),7.17(s,1H),6.54 -6.77(m,3H),5.76(d,J=8.8Hz,1H),3.94(s,3H),3.12(t,J=5.2Hz,2H),2.80(t,J=5.2Hz,2H) ,2.68(s,3H),2.56(s,6H).LC-MS:m / z 471[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com