Pyrimido diazepine compound as well as pharmaceutical composition and application thereof
A technology of diazepines and compounds, applied in the field of pyrimidodiazepines and their pharmaceutical compositions, which can solve problems such as poor selectivity, clinical pressure of drug resistance, wild-type cell toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
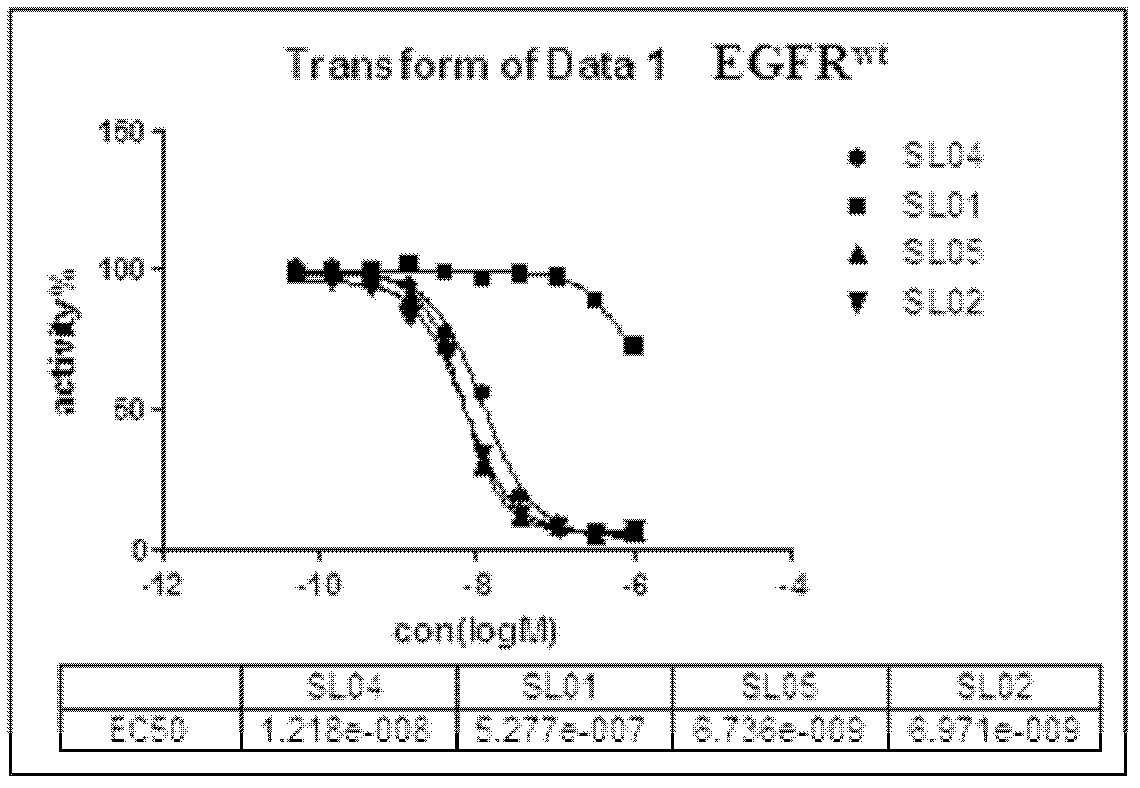
[0211] N-(3-(2-(2-methoxy-4-(4-methylpiperazine-1-substituted)aniline)-6-methyl-5,8-dioxo-7,8- Dihydro-5H-pyrimido[4,5-e][1,4]diazatable-9(6H)-substituted)phenyl)acrylamide (SL02)
[0212] (N-(3-(2-(2-methoxy-4-(4-methylpiperazin-1-yl)phenylamino)-6-methyl-5,8-dioxo-7,8-dihydro-5H-pyrimido[4, 5-e][1,4]diazepin-9(6H)-yl)phenyl)acrylamide)
[0213]
[0214] Step 1. Ethyl 4-(3-tert-butoxycarbonylaminoaniline)-2-methylmercaptopyrimidine-5-carboxylate (1)
[0215] (ethyl 4-(3-(tert-butoxycarbonylamino)phenylamino)-2-(methylthio)pyrimidine-5-carboxylate)
[0216]
[0217] 4-Chloro-2-methylmercaptopyrimidine-5-ethyl carbonate (12.63g, 54.27mmol), N-Boc m-phenylenediamine (11.29g, 54.27mmol), K 2 CO 3 (15g, 108.54mmol) was dissolved in 200ml of anhydrous DMF, heated to 80°C under the protection of argon, and stirred overnight. After cooling to room temperature, 500 ml of ice water was added with stirring, and a large amount of solids precipitated out. It was filtered under...
Embodiment 2
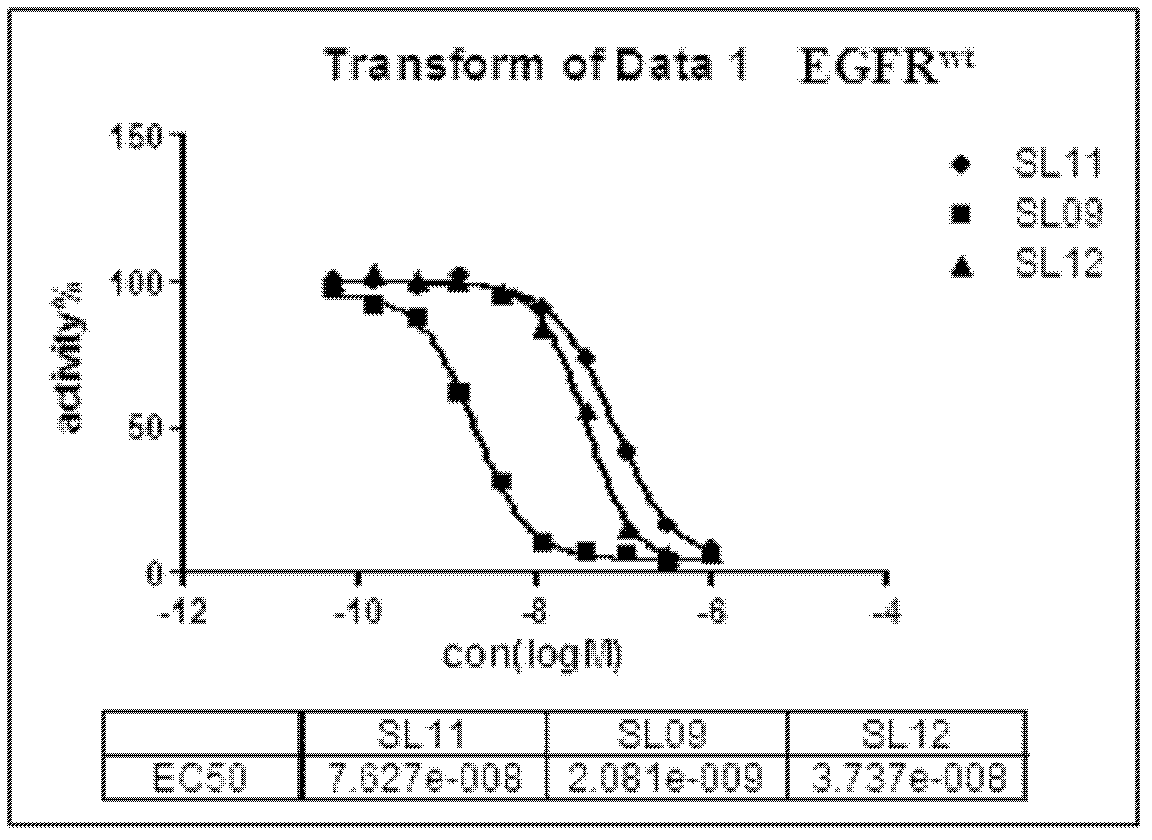
[0267] N-(3-(2-(2-methoxy-4-(4-methylpiperazine-1-substituted)anilino)-5,10-dioxo-8,9,9a,10-tetra Hydrogen-5H-pyrimido[4,5-e]pyrrolo[1,2-a][1,4]diazatable-11(7H)-substituted)phenyl)acrylamide (SL01)
[0268] N-(3-(2-(2-methoxy-4-(4-methylpiperazin-1-yl)phenylamino)-5,10-dioxo-8,9,9a,10-tetrahydro-5H-pyrimido[4,5 -e]pyrrolo[1,2-a][1,4]diazepin-11(7H)-yl)phenyl)acrylamide
[0269]
[0270] The synthesis method is as in Example 1.
[0271] 1 H NMR (400MHz, CDCl 3 )δ8.95(s, 1H), 7.91(s, 1H), 7.78(s, 1H), 7.73(s, 1H), 7.44(t, J=7.6Hz, 1H), 7.38(s, 1H), 7.00(s, 1H), 6.93(d, J=7.2Hz, 1H), 6.42-6.38(m, 2H), 6.22-6.16(m, 1H), 6.09(s, 1H), 5.74(d, J= 10.0Hz, 1H), 4.34(d, J=5.2Hz, 1H), 3.80(s, 3H), 3.77(s, 1H), 3.67-3.65(m, 1H), 3.12(br, 4H), 2.79( s, 1H), 2.60 (br, 4H), 2.38 (s, 3H), 2.14-2.05 (m, 3H).
[0272] 13 C NMR (125MHz, CDCl 3 )δ169.16,163.27,162.40,159.46,158.28,148.79,147.51,140.23,139.17,131.05,130.10,127.96,124.35,120.46,120.31,119.64,119.53,113.04,110.43,107.76...
Embodiment 3
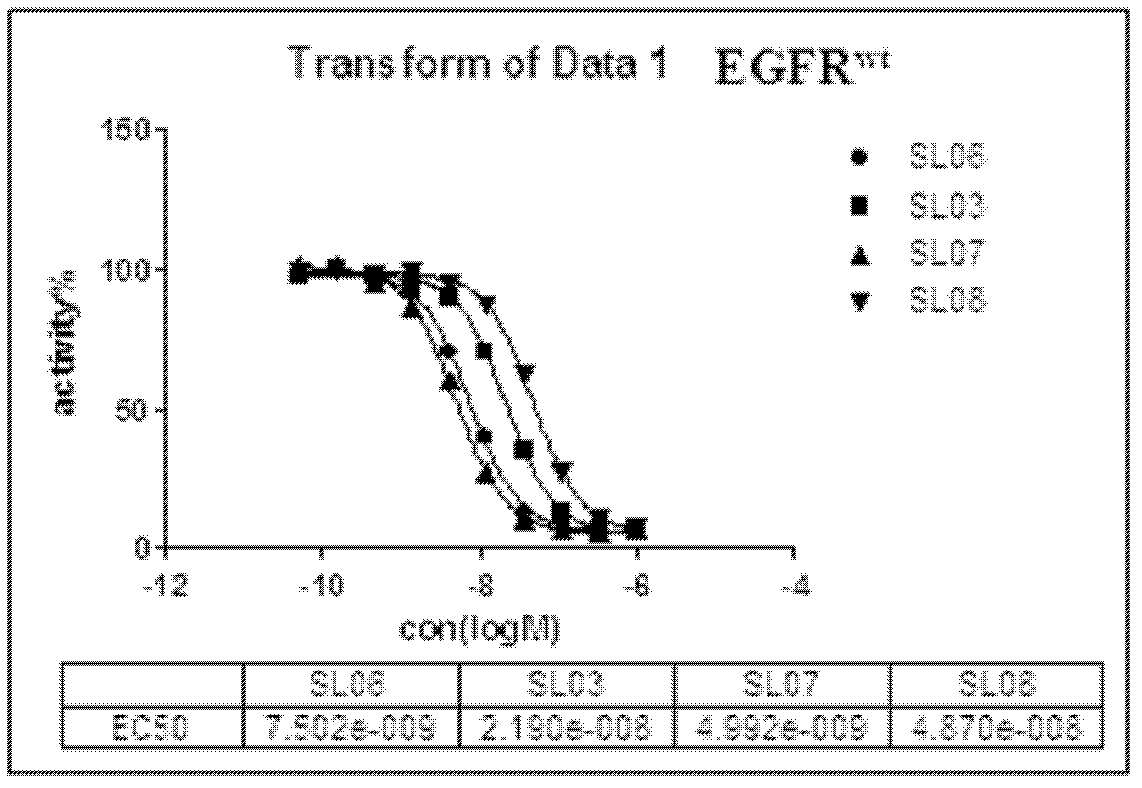
[0275] N-(3-(2-(2-methoxy-4-(4-methylpiperazine-1-substituted)anilino)-5,11-dioxo-7,8,9,10,10a , 11-hexahydropyrrolo[1,2-a]pyrimido[4,5-e][1,4]diazatable-12(5H)-substituted)phenyl)acrylamide (SL09)
[0276] N-(3-(2-(2-methoxy-4-(4-methylpiperazin-1-yl)phenylamino)-5,11-dioxo-7,8,9,10,10a,11-hexahydropyrido[1, 2-a]pyrimido[4,5-e][1,4]diazepin-12(5H)-yl)phenyl)acrylamide
[0277]
[0278] The synthesis method is as in Example 1.
[0279] 1 H NMR (400MHz, CDCl 3 )δ8.87(s, 1H), 7.97(s, 1H), 7.90(s, 1H), 7.77(s, 1H), 7.43(t, J=8.0Hz, 1H), 7.40(s, 1H), 7.08(s, 1H), 6.92(d, J=7.6Hz, 1H), 6.40-6.36(m, 2H), 6.27-6.20(m 1H), 6.09(s, 1H), 5.72(d, J=10.8 Hz, 1H), 4.50-4.47(m, 1H), 4.43-4.40(m, 1H), 3.79(s, 3H), 3.15(br, 4H), 3.06-2.99(m, 1H), 2.67(br, 4H), 2.42 (s, 3H), 2.27 (s, 1H), 1.95-1.90 (m, 1H), 1.83-1.62 (m, 4H).
[0280] 13 C NMR (125MHz, CDCl 3 )δ169.68,166.07,163.26,162.49,159.44,158.26,148.78,147.61,139.42,139.06,131.02,130.05,127.95,124.47,120.39,120.33,119.70,119...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com