Benzodihydropyrone derivative and preparation method thereof
A technology for chromones and derivatives, applied in organic chemistry and other directions, can solve problems such as low yield, and achieve the effects of high yield, easy separation, and large application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
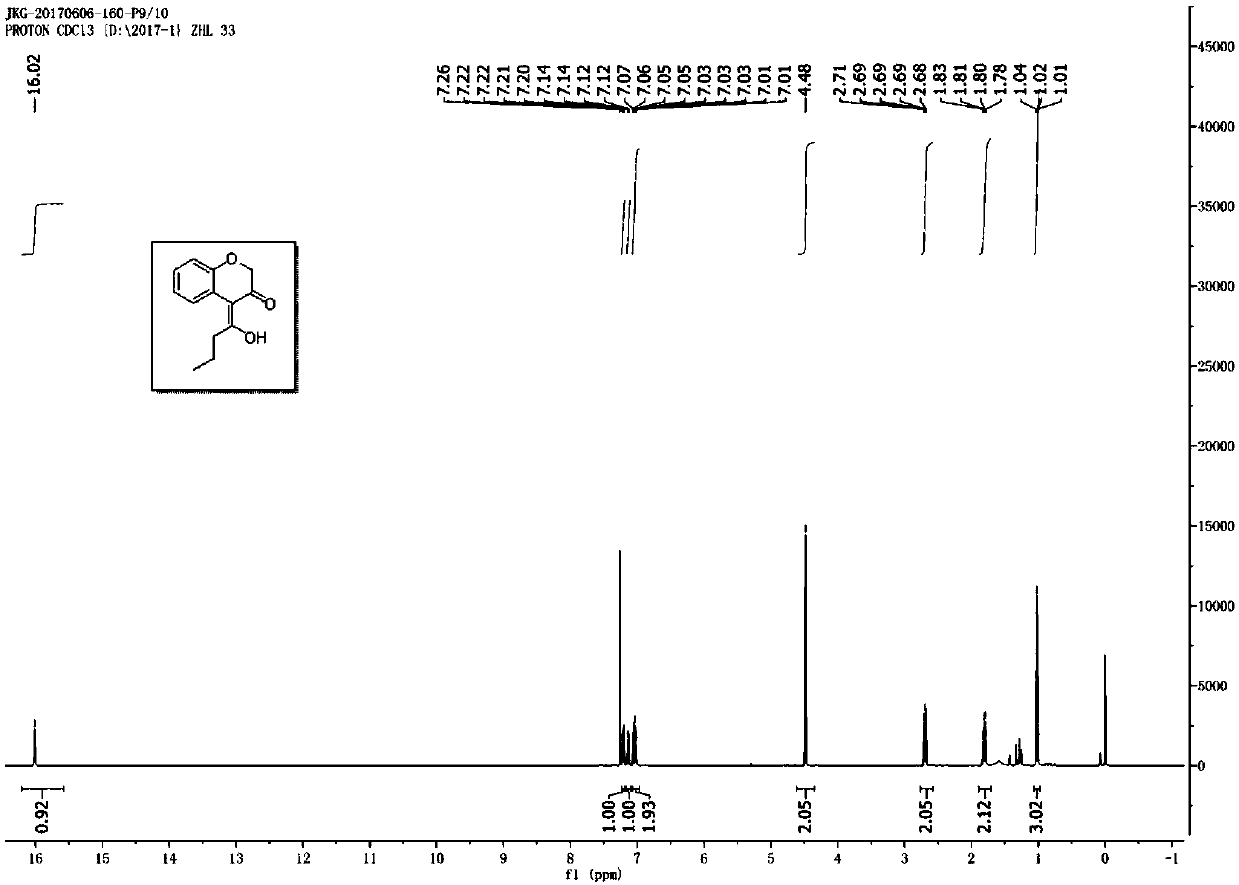
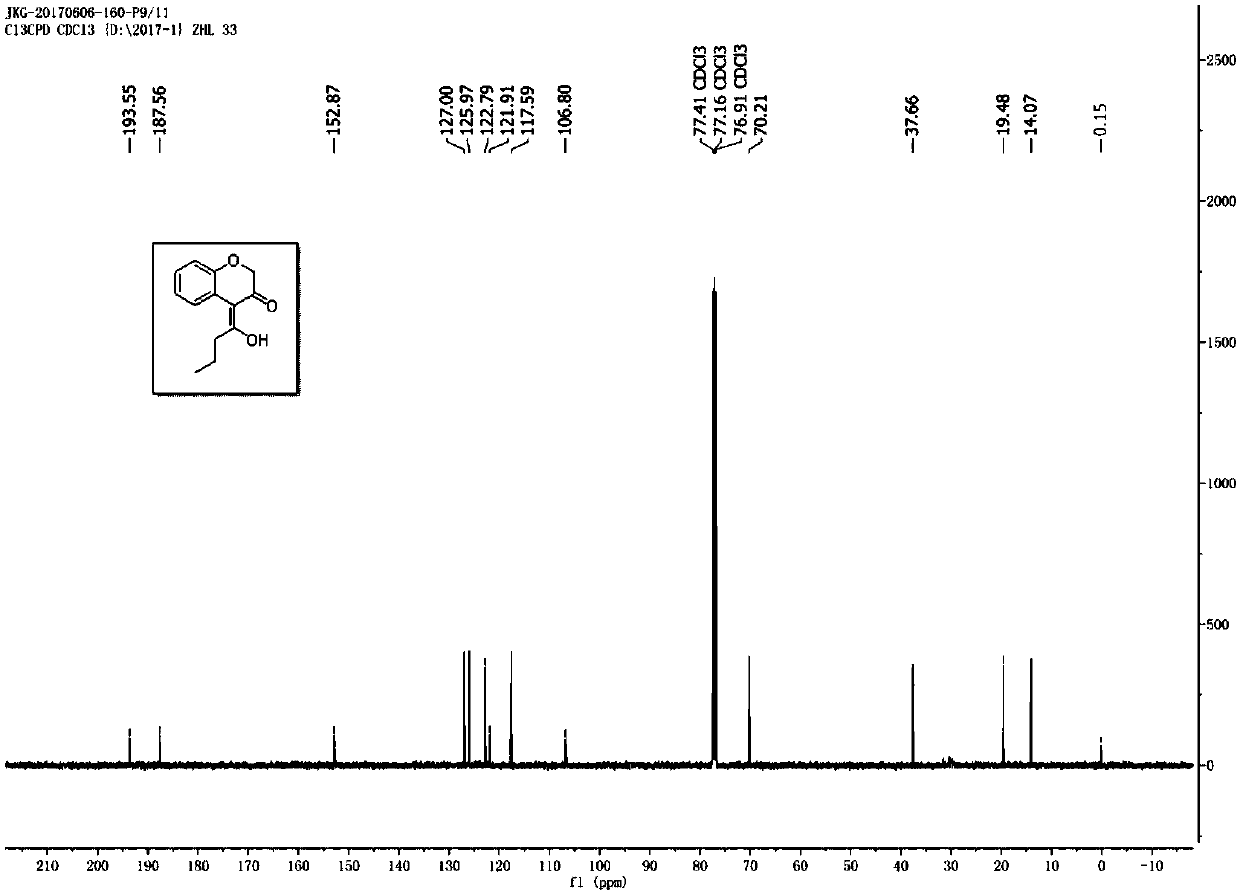
[0028] Prepare a dry reaction tube, dissolve 1-phenoxyhept-2-yn-4-one (0.2mmol) in 4mL chlorobenzene, add gold picolinate (PicAuCl 2 ) (3.9 mg), 2,6-dichloropyridine nitrogen oxide (49 mg), and then the reaction system was placed at 60 ° C for 2.5 h, and after separation by chromatographic column, chromanones were obtained Derivatives, the appearance of the above-mentioned chromanone derivatives is a colorless oil, and its yield is 78%; the chemical structure of the above-mentioned products is detected by nuclear magnetic resonance, as figure 1 As shown, its hydrogen spectrum 1 H NMR (500MHz, CDCl 3 ):δ16.02(s,1H),7.20(dd,J=7.5,1.5Hz,2H),7.12(dd,J=7.0,1.0Hz,1H),7.07-7.01(m,2H),4.48( s, 2H), 2.69(t, J=7.0Hz, 2H), 1.82-1.78(m, 2H), 0.1.02(t, J=7.0Hz, 3H); figure 2 As shown, its carbon spectrum 13 C NMR (125MHz, CDCl 3 ): δ193.54,187.55,152.85,126.98,125.95,122.77,121.88,117.57,106.79,70.18,37.64,19.47,14.06. The structural formula is as follows:
[0029]
Embodiment 2
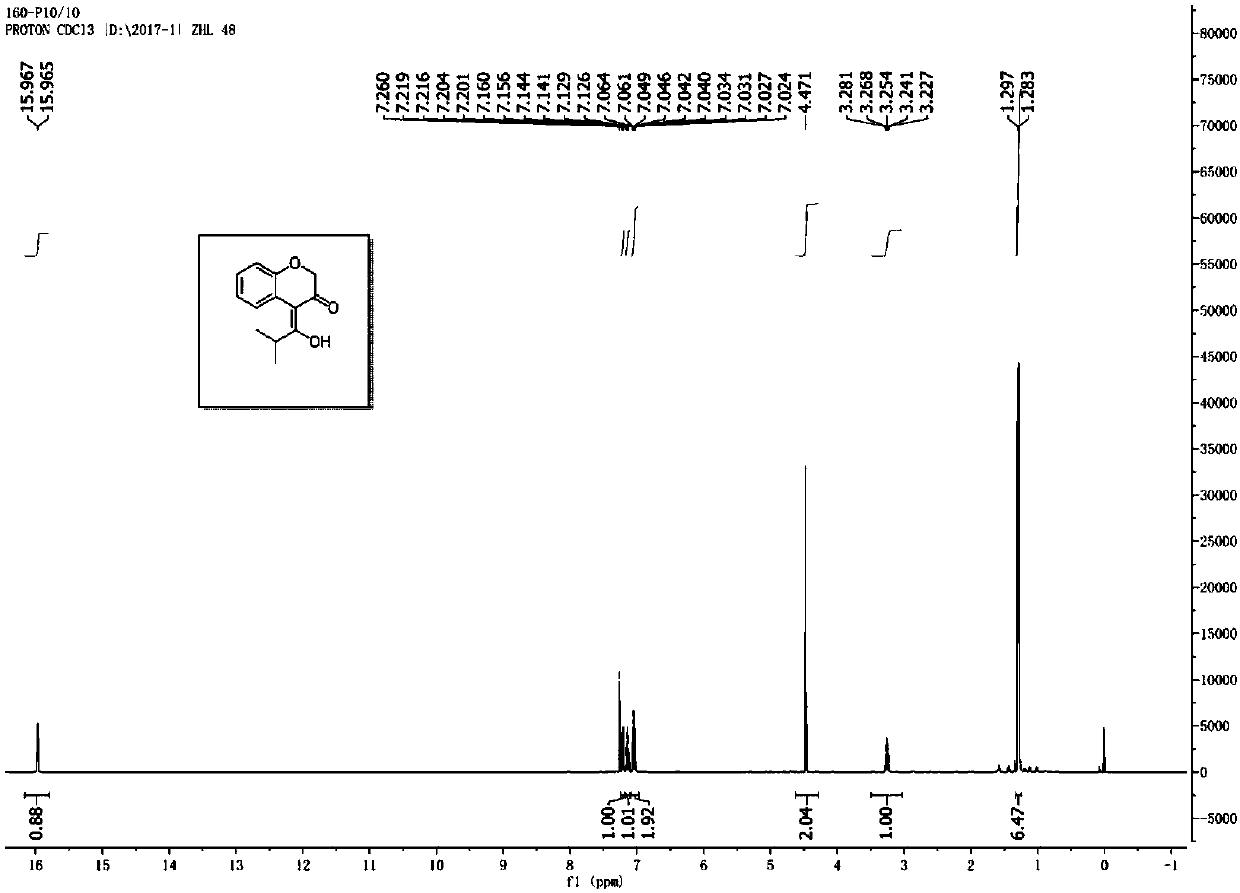
[0031] Prepare a dry reaction tube, dissolve 2-methyl-6-phenoxyhex-4-yn-3-one (0.2mmol) in 4mL chlorobenzene, add PicAuCl successively at room temperature 3 (3.9mg), 2,6-dichloropyridine nitrogen oxide (49mg), and then the reaction system was placed at 60oC for 2.5h, and the substituted chromanone derivatives could be obtained through chromatography column separation; The appearance of the above-mentioned chromanone derivatives is a colorless oil, and its yield is 64%; the chemical structure of the above-mentioned product is detected by nuclear magnetic resonance method, as shown in image 3 As shown, its hydrogen spectrum 1 H NMR (500MHz, CDCl 3 ):δ15.97(s,1H),7.21(dd,J=7.5,1.5Hz,2H),7.12(dt,J=8.0,2.0Hz,1H),7.06-7.02(m,2H),4.47( s,2H),3.28-3.23(m,1H),1.28(d,J=7.0Hz,6H); Figure 4 As shown, its carbon spectrum 13 C NMR (125MHz, CDCl 3 ): δ194.92, 191.04, 153.03, 127.03, 125.90, 122.89, 121.97, 117.68, 105.47, 70.48, 32.36, 19.96; the above-mentioned proton NMR spectrum an...
Embodiment 3
[0034] Prepare a dry reaction tube, dissolve 4-phenoxy-1-phenylbut-2-yn-1-one (0.2mmol) in 4mL chlorobenzene, add PicAuCl successively at room temperature 2 (3.9mg), 2,6-dichloropyridine nitrogen oxide (49mg), and then the reaction system was placed at 60oC for 2.0h, and the substituted chromanone derivatives could be obtained through chromatography column separation; The appearance of the above-mentioned chromanone derivatives is a colorless oil, and its yield is 94%; the chemical structure of the above-mentioned product is detected by nuclear magnetic resonance method, as shown in Figure 5 As shown, its hydrogen spectrum 1 H NMR (500MHz, CDCl 3 ):δ15.68(s,1H),7.58(d,J=7.0Hz,2H),7.49(dt,J=8.0,1.5Hz,1H),7.39-7.36(m,2H),7.05-6.99( m,2H),6.69-6.62(m,2H),4.58(s,2H); Image 6 As shown, its carbon spectrum 13 C NMR (125MHz, CDCl 3 ): δ193.78, 181.50, 152.52, 135.34, 131.90, 129.04, 128.60, 127.48, 127.02, 122.22, 121.76, 117.61, 105.76, 70.02; Its chemical structural formula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com