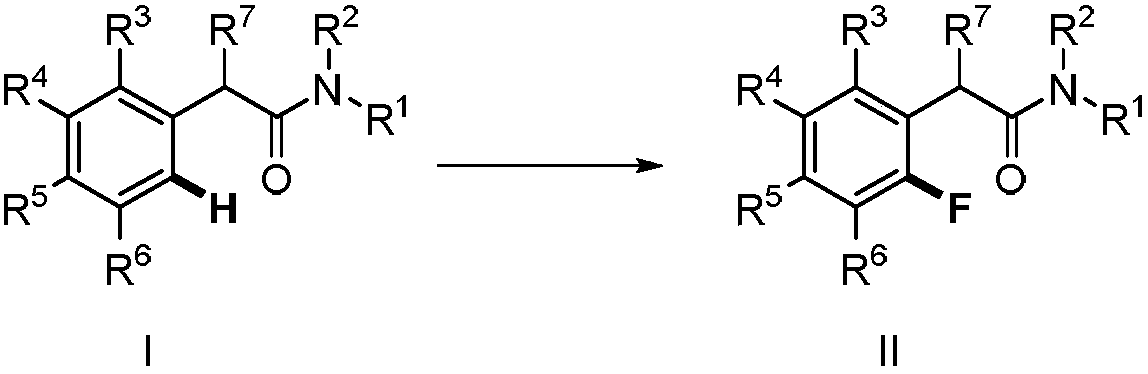
Method for synthesizing 2-fluoro-N-substituted aryl acetamide compound
A technology of aryl acetamide and fluorobisbenzenesulfonamide, which is applied in organic chemical methods, chemical instruments and methods, organic halogenation, etc., can solve problems such as low efficiency and unrealized C-H activation, and achieve wide substrate adaptability , high atomic utilization, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
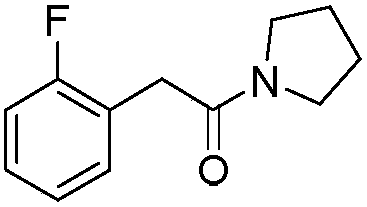
[0036] Add N-substituted phenylacetamide (37.8mg, 0.2mmol), palladium chloride (3.6mg, 0.02mmol), N-fluorobenzenesulfonimide (126.1mg, 0.4mmol) into a closed reaction vessel, Silver nitrate (13.6mg, 0.08mmol), 1,2-dichloroethane (2.0mL), and the reaction mixture was stirred at 45°C, followed by TLC detection, and the reaction was complete within 24 hours. The reaction was stopped, the mixture was diluted with ethyl acetate, the solvent was removed under reduced pressure, and the residue was subjected to column chromatography [GF254 silica gel; 200-300 mesh; the developing solvent was V (petroleum ether) / V (ethyl acetate) = 3 / 1] After separation and purification, the eluate containing the product was collected, and the solvent was evaporated from the eluate to obtain 33.1 mg of pure 2-fluoro-N-substituted phenylacetamide, with a yield of 80%.
Embodiment 2
[0038]
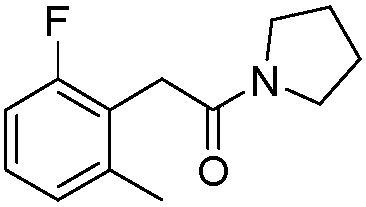
[0039] Add 2-methyl-N-substituted phenylacetamide (40.6mg, 0.2mmol), palladium chloride (3.6mg, 0.02mmol), N-fluorobenzenesulfonimide (126.1mg , 0.4mmol), silver nitrate (13.6mg, 0.08mmol), 1,2-dichloroethane (2.0mL), the reaction mixture was stirred and reacted at 60°C, followed by TLC detection, and the reaction was complete within 24h. The reaction was stopped, the mixture was diluted with ethyl acetate, the solvent was removed under reduced pressure, and the residue was subjected to column chromatography [GF254 silica gel; 200-300 mesh; the developing solvent was V (petroleum ether) / V (ethyl acetate) = 3 / 1] After separation and purification, the eluent containing the product was collected, and the solvent was evaporated from the eluent to obtain 40.2 mg of pure 6-fluoro-2-methyl-N-substituted phenylacetamide, with a yield of 91%.
Embodiment 3
[0041]
[0042] Add m-methyl-N-substituted phenylacetamide (40.6mg, 0.2mmol), palladium chloride (3.6mg, 0.02mmol), N-fluorobenzenesulfonimide (126.1mg, 0.4mmol), silver nitrate (13.6mg, 0.08mmol), 1,2-dichloroethane (2.0mL), the reaction mixture was stirred and reacted at 45°C, followed by TLC detection, and the reaction was complete within 24h. The reaction was stopped, the mixture was diluted with ethyl acetate, the solvent was removed under reduced pressure, and the residue was subjected to column chromatography [GF254 silica gel; 200-300 mesh; the developing solvent was V (petroleum ether) / V (ethyl acetate) = 3 / 1] After separation and purification, the eluent containing the product was collected, and the solvent was evaporated from the eluent to obtain 35.8 mg of pure 2-fluoro-5-methyl-N-substituted phenylacetamide, with a yield of 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com