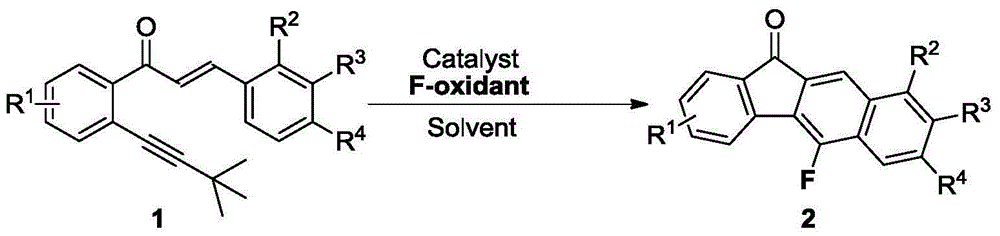
Method for synthesizing fluorofluorenone compound
A technology of fluorinated fluorenones and compounds, which is applied in the field of preparation of fluorinated fluorenones, can solve the problems of expensive transition metal catalysts, toxicity, etc., and achieve the effects of good substrate adaptability, simple reaction steps, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
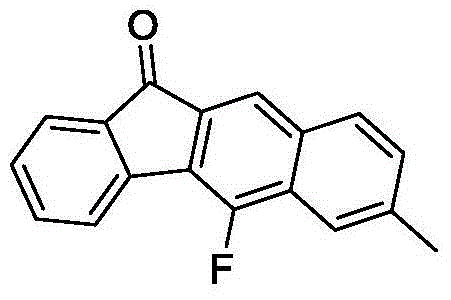
[0037] Mix 0.3mmol (E)-1-(2'-tert-butylethynyl)-3-p-tolyl-2-allyl ketone (90.6mg), 0.6mmol Selectfluor (212.4mg) and 0.015mmol Cu powder (0.96mg ) was added to a 15mL thick-walled pressure-resistant reaction tube, and then 3mL of acetonitrile was added as a solvent. Next, magnetic stirring was performed at 50° C. for 1.5 hours. After cooling to room temperature, two spoonfuls of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and then separated by column chromatography, using petroleum ether / dichloromethane=5:1 as eluent, collect the eluent containing the product, evaporate the solvent from the eluent to obtain 7-methyl-5-fluoro-11H-benzofluorenone. The material was a yellow solid in 70% yield.
[0038] Characterization data: IR(KBr,cm -1 ):ν=1705 (C=O); 1 H NMR (500MHz, CDCl 3 ): δ7.97(s,1H),7.91(d,J=7.5Hz,1H),7.88(s,1H),7.81(d,J=8.0Hz,1H),7.77(d,J=7.5Hz ...
Embodiment 2
[0040]
[0041]0.3 mmol (E)-1-(2'-tert-butylethynyl)-3-p-tolyl-2-allylone (90.6 mg), 0.4 mmol Selectfluor (141.7 mg) and 0.015 mmol Cu (0.96 mg) Add the powder into a 15mL thick-walled pressure-resistant reaction tube, and then add 3mL DMF as a solvent. Next, magnetic stirring was performed at 60° C. for 1.5 hours. After cooling to room temperature, two spoonfuls of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and then separated by column chromatography, using petroleum ether / dichloromethane=5:1 as eluent, collect the eluate containing the product, evaporate the solvent from the eluate to obtain 7-methyl-5-fluoro-11H-benzofluorenone. The material was a yellow solid, 60% yield.
[0042] Characterization data: IR(KBr,cm -1 ):ν=1705 (C=O); 1 H NMR (500MHz, CDCl 3 ): δ7.97(s,1H),7.91(d,J=7.5Hz,1H),7.88(s,1H),7.81(d,J=8.0Hz,1H),7.77(d,J=7.5Hz ,1H),7.60(t,J=7.5Hz,1H),...
Embodiment 3
[0044]
[0045] 0.3mmol (E)-1-(2'-tert-butylethynyl)-3-p-tolyl-2-allyl ketone (90.6mg), 0.6mmol N-fluoropyridine trifluoromethanesulfonate (147.6mg) and 0.015 mmol CuCN (1.35 mg) powder were added to a 15 mL thick-walled pressure-resistant reaction tube, and then 3 mL of acetonitrile was added as a solvent. Next, magnetic stirring was performed at 45° C. for 2.5 hours. After cooling to room temperature, two spoonfuls of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and then separated by column chromatography, using petroleum ether / dichloromethane=5:1 as eluent, collect the eluate containing the product, evaporate the solvent from the eluate to obtain 7-methyl-5-fluoro-11H-benzofluorenone. The material was a yellow solid, 68% yield.
[0046] Characterization data: IR(KBr,cm -1 ):ν=1705 (C=O); 1 H NMR (500MHz, CDCl 3 ): δ7.97(s,1H),7.91(d,J=7.5Hz,1H),7.88(s,1H),7.81...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com