Nucleic acid containing photosensitive unit, preparation method and application thereof
A photosensitive unit and nucleic acid technology, which is applied in the preparation of sugar derivatives, chemical instruments and methods, organic chemistry, etc., can solve the problems of cumbersome operation, poor efficiency, and mild reaction, and achieve convenient operation, simplified methods, and reduced preparation costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
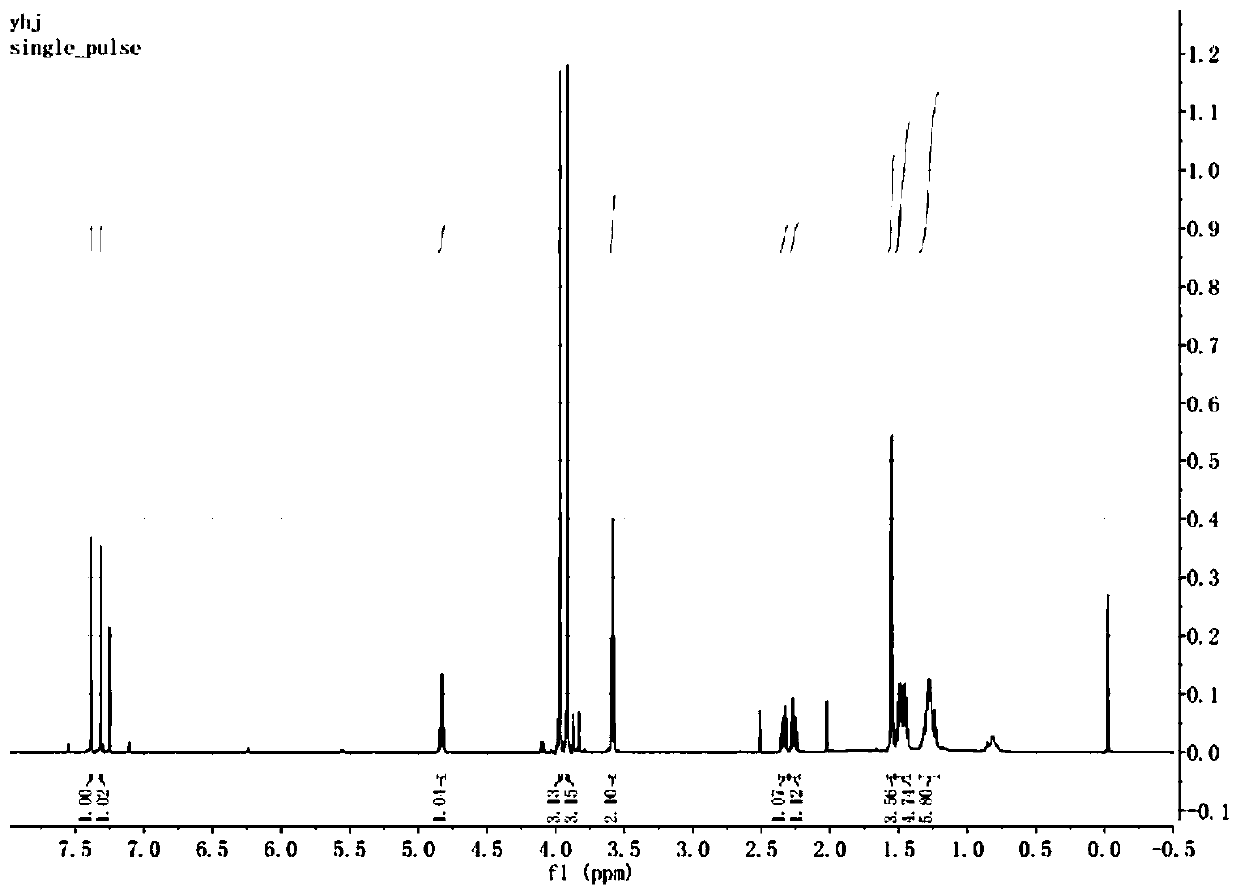
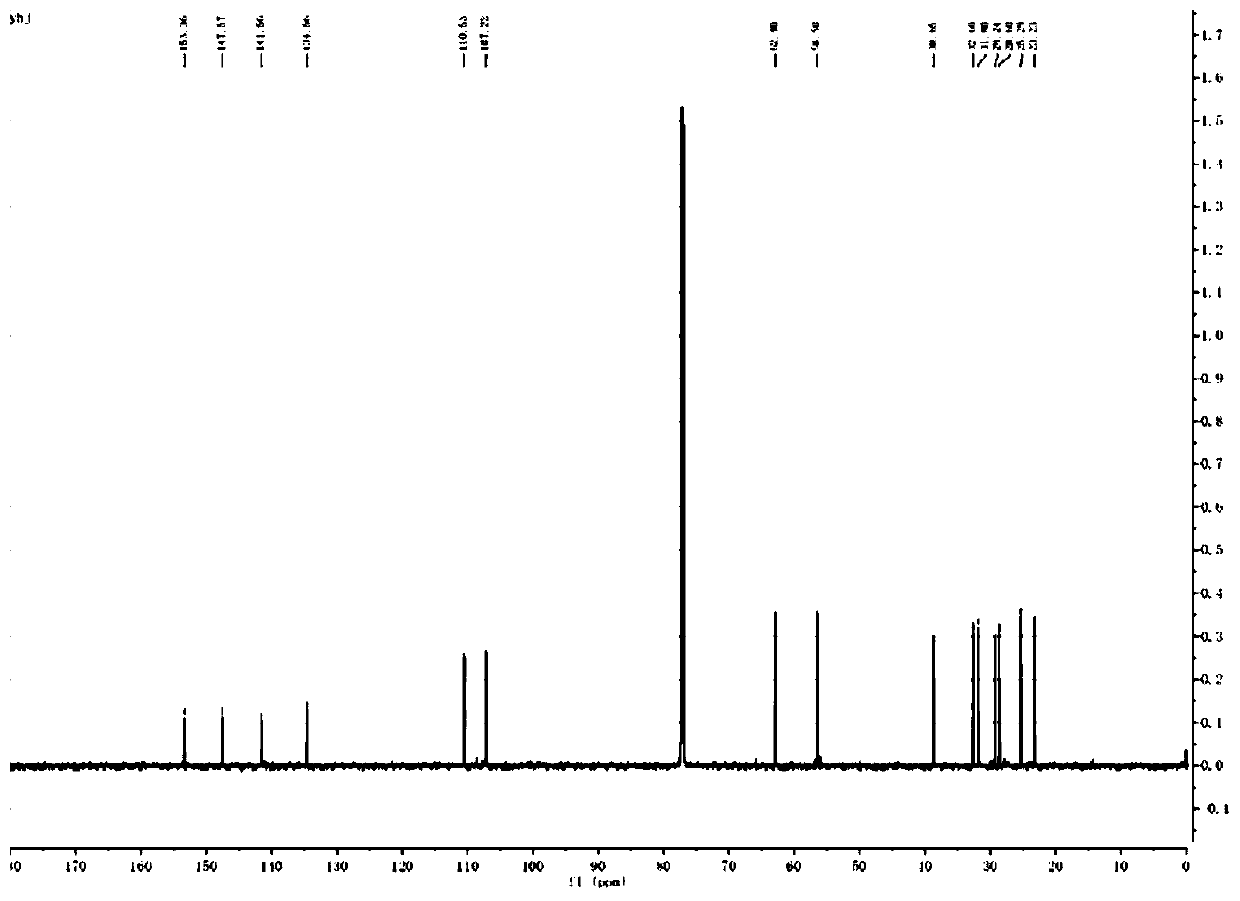
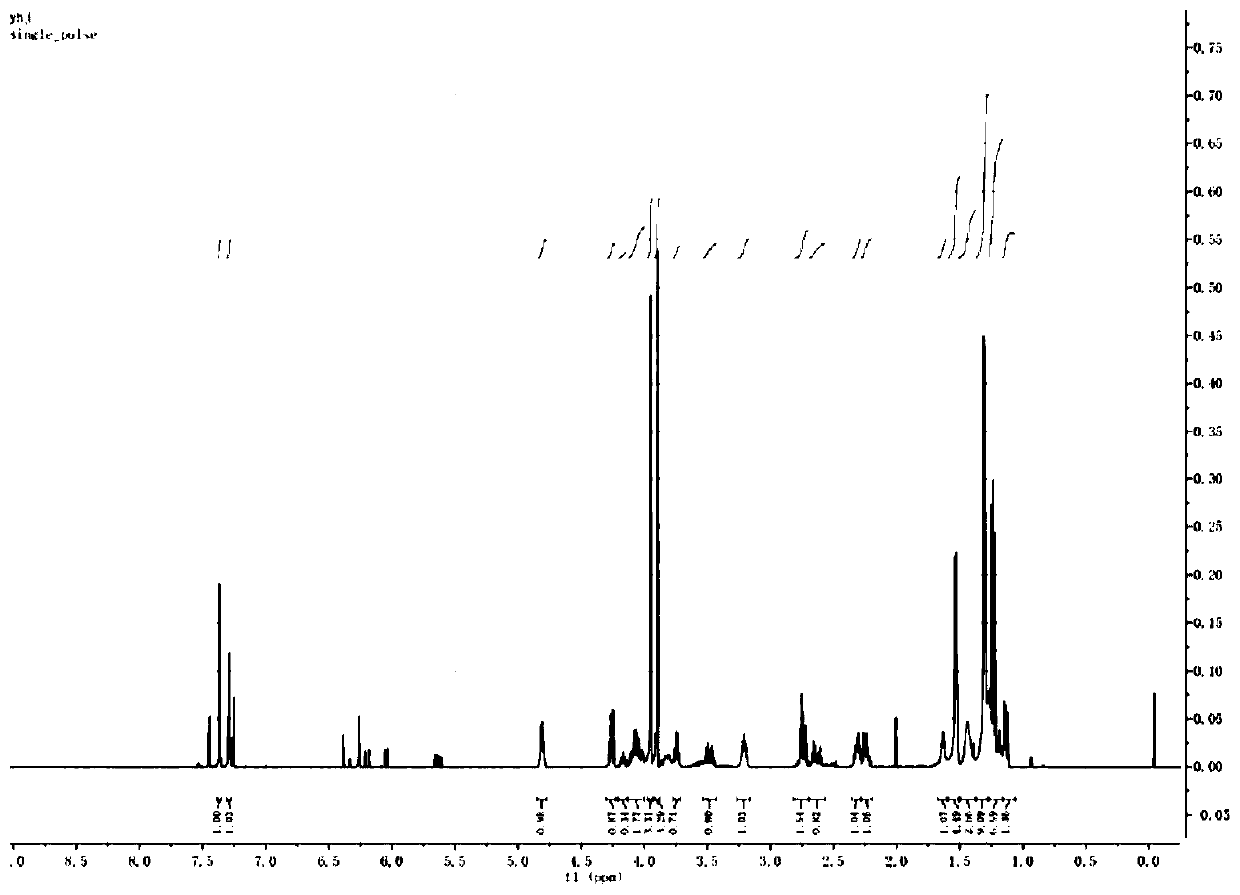
Image
Examples
preparation example Construction
[0036] An embodiment of the present invention provides a method for preparing a nucleic acid containing a photosensitive unit according to the above embodiments, comprising the following steps:
[0037] S1: Preparation of sulfur-containing phosphoroimide ester: starting from n-mercapto-1-alkanol, it was mixed with 4,5-dimethoxy-2-nitrophenyl-ethyl chloride Under the action of an organic base, the reaction generates n-(4,5-dimethoxy-2-nitrophenyl)-ethylsulfide-1-alkanol, which is then combined with 2-cyanoethyl-N, N-diisopropyl phosphoramidite chloride reacts under the action of an organic base to generate n-(4,5-dimethoxy-2-nitrophenyl)-ethylsulfide-1-(2-cyano Ethyl-N,N-diisopropylphosphoramidite alkyl alcohol ester) to obtain sulfur-containing phosphoramidite;
[0038] In this step, the obtained sulfur-containing phosphoramidite has a structural formula as shown in formula (II):
[0039]
[0040] Wherein, the range of n value is 1-15.
[0041]S2: Preparation of nucleic ...
Embodiment 1
[0065] The preparation method of sulfur-containing phosphoramidite comprises the following steps:
[0066] Step 1: Preparation of 4,5-dimethoxy-2-nitrophenyl-ethyl chloride
[0067] 50 mg of ferric chloride hexahydrate was dispersed in 200 ml of ethanol, and 1.0 g of powdered activated carbon was added at one time under stirring in an ice bath, and stirred at room temperature for 2 hours. Subsequently, 10 ml of 35% hydrazine hydrate and 4.0 g of 4,5-dimethoxy-2-nitroacetophenone were added, the reaction solution was stirred at room temperature for 1 hour, and 20 ml of 1M hydrochloric acid solution was added dropwise thereto, and the insoluble thing. After the filtrate was concentrated, it was redissolved in 50 ml of ethyl acetate, washed with 200 ml of saturated brine, and the organic phase was separated and dried over anhydrous sodium sulfate. The organic phase was rotary evaporated to obtain 4.0 g of (4,5-dimethoxy-2-nitrophenyl)-ethanol (light yellow solid), with a yield ...
Embodiment 2
[0080] A method for preparing a nucleic acid containing a photosensitive unit, comprising the following steps:
[0081] Step 1: Take 700 mg of 6-(4,5-dimethoxy-2-nitrophenyl)-ethylsulfide-1-(2-O-cyanoethyl-N,N-diisopropyl Phosphoramidite hexanol ester) was dissolved in 10 ml of anhydrous acetonitrile, and transferred to the end group modification reagent bottle of ABI 394 nucleic acid synthesizer under nitrogen protection. Input nucleic acid sequence: 5'CCT AGA TTC AGT TCA ACT TA 3', use DMT-retained synthesis mode, and perform synthesis on the order of 1 μmol.
[0082] Step 2: After the synthesis, the solid phase carrier is taken out, dispersed in 1 ml of 5%-32% methylammonia aqueous solution, heated at 55-75°C for 1-4 hours, centrifuged to remove insoluble matter, and the solution is concentrated to obtain the crude product . The target product was separated by high performance liquid chromatography and characterized by MALDI-TOF for structural information.
[0083] The p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com