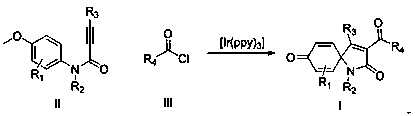Preparation method of 3-acyl screw ring dibenzosuberenone compound
A kind of technology of acyl spirocyclic trienone and compound, which is applied in the field of preparation of 3-acyl spirocyclic trienone compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-17
[0036] Embodiment 1-17 Reaction condition optimization:
[0037] Using alkyne amide and benzoyl chloride shown in formula three as raw materials, the influence of various conditions on the reaction (formula two) was explored, and representative examples were selected. The results are shown in table one:
[0038] Example
Reaction condition (variable)
Yield (%)
1
---
88
2
[Ru(bpy) 3 Cl 2 ] instead of [Ir(ppy) 3 ]
0
3
Eosin Y instead of [Ir(ppy) 3 ]
0
4
Do not join [Ir(ppy) 3 ]
0
5
in the dark
0
6
[Ir(ppy) 3 ] Feed amount is replaced by 5 mol %
89
7
36W fluorescent lamp replaces the LED lamp in Example 1
73
8
Na 2 CO 3 Instead of 2,6-lutidine
53
9
K 2 CO 3 Instead of 2,6-lutidine
47
10
Et 3 N instead of 2,6-lutidine
51
11
Toluene instead of CH 3 CN
32
12
BuOAc instead of CH 3 CN
61
...
Embodiment 18
[0046] Example 18 target product Synthesis
[0047] Add a magnetic stirring bar to the reactor, add the corresponding alkyne amide compound (0.2mmol) (refer to the reaction formula of formula 1 for the structure), photocatalyst [Ir(ppy) 3 ] (2mol%) and 2,6-lutidine (2.0 equivalents), then add benzoyl chloride (0.4mmol) and organic solvent acetonitrile (2mL), then replace the air in the reactor with argon to replace 3- After 5 times, the reactor was then placed in an oil bath equipped with magnetic stirring at a temperature of 100° C., and heated for 24 hours under the light condition of a 5W blue LED lamp as the light source. After the reaction was complete, the solvent was evaporated to dryness with a rotary evaporator, and the residue was separated and purified with a chromatographic column to obtain the target product with a yield of 83% ( 1 H NMR (400 MHz, CDCl 3 ): 7.84 (d, J = 7.6 Hz, 2H), 7.52(t, J = 8.0 Hz, 1H), 7.38 (t, J = 8.0 Hz, 2H), 7.23 (s, 5H), 7.22-7....
Embodiment 19
[0048] Example 19 target product Synthesis
[0049] Add a magnetic stirring bar to the reactor, add the corresponding alkyne amide compound (0.2mmol) (refer to the reaction formula of formula 1 for the structure), photocatalyst [Ir(ppy) 3 ] (2mol%) and 2,6-lutidine (2.0 equivalents), then add benzoyl chloride (0.4mmol) and organic solvent acetonitrile (2mL), then replace the air in the reactor with argon to replace 3- After 5 times, the reactor was then placed in an oil bath equipped with magnetic stirring at a temperature of 100° C., and heated for 24 hours under the light condition of a 5W blue LED lamp as the light source. After the reaction was complete, the solvent was evaporated to dryness with a rotary evaporator, and the residue was separated and purified with a chromatographic column to obtain the target product with a yield of 67% ( 1 H NMR (400 MHz, CDCl 3 ): 7.85 (t, J = 4.0 Hz, 2H), 7.54(d, J = 8.0 Hz, 1H), 7.41 (t, J = 8.0 Hz, 2H), 7.28-7.20 (m, 5H), 6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com