Synthesis method for iodoisoxazoline compound
A synthetic method and isoxazoline technology, which is applied in the field of synthesis of iodoisoxazoline compounds, can solve problems such as low utilization rate of atoms, expensive metal catalysts, and complicated operation conditions, and achieve low-cost, environment-friendly and low-cost reagents. Good material adaptability and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
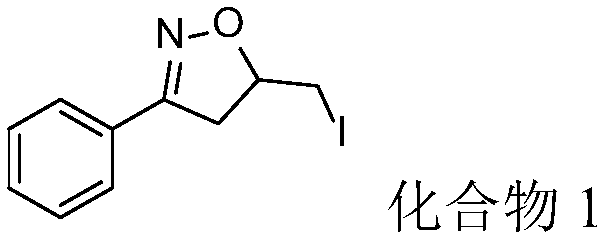
[0032] 1-Phenylbut-3-en-1-one oxime (40.3, 0.25mmol), elemental iodine (31.8mg, 0.125mmol) and tert-butyl hydroperoxide (64.4mg, 0.5mmol, 70%wt aqueous solution) Join in the argon protection reaction bottle, add water 2ml again finally, react at room temperature 15h again, after the reaction finishes, use column chromatography (eluent: sherwood oil / ethyl acetate volume ratio 10:1) to separate Compound 1 (57.4 mg) was obtained with a yield of 80%.
[0033] Product characterization: white solid; m.p.72-73°C; 1 H NMR (500MHz, CDCl 3 )δ7.71–7.63(m,2H),7.48–7.38(m,3H),4.95–4.89(m,1H),3.52(dd,J=17.0,10.4Hz,1H),3.42(dd,J= 10.0,4.1Hz,1H),3.28–3.18(m,2H).
Embodiment 2
[0035]
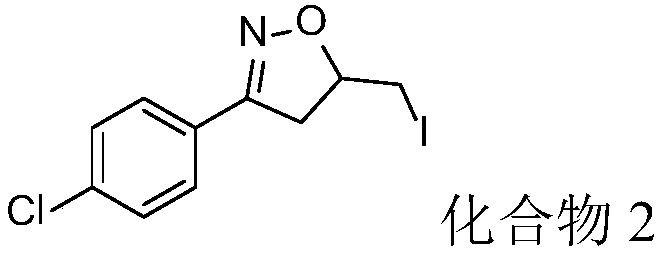
[0036] 1-(4-Chlorophenyl)but-3-en-1-one oxime (48.9, 0.25mmol), elemental iodine (31.8mg, 0.125mmol) and tert-butyl hydroperoxide (64.4mg, 0.5mmol, 70%wt aqueous solution) joins in the argon protection reaction bottle, finally adds water 2ml again, reacts 15h at room temperature again, after reaction finishes, with column chromatography (eluent: sherwood oil / ethyl acetate volume ratio 10:1) Compound 2 (67.5 mg) was isolated with a yield of 84%.
[0037] Product characterization: white solid: m.p.98-99°C; 1 H NMR (500MHz, CDCl 3)δ7.65–7.55(m,2H),7.46–7.31(m,2H),4.96–4.90(m,1H),3.49(dd,J=17.0,10.4Hz,1H),3.42(dd,J= 10.1,4.0Hz,1H),3.29–3.10(m,2H).
Embodiment 3
[0039]
[0040] 1-(p-tolyl)but-3-en-1-one oxime (43.8, 0.25mmol), elemental iodine (31.8mg, 0.125mmol) and tert-butyl hydroperoxide (64.4mg, 0.5mmol, 70% wt aqueous solution) was added in the argon-protected reaction flask, and finally 2ml of water was added, and then reacted at room temperature for 15h. After the reaction, column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 10: 1) Compound 3 (48.2 mg) was isolated with a yield of 64%.
[0041] Product characterization: white solid; m.p.96–97°C; 1 H NMR (500MHz, CDCl 3 )δ7.61–7.50(m,2H),7.22(d,J=8.0Hz,2H),4.94–4.88(m,1H),3.50(dd,J=17.0,10.3Hz,1H),3.42(dd ,J=10.0,4.1Hz,1H),3.27–3.16(m,2H),2.38(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com