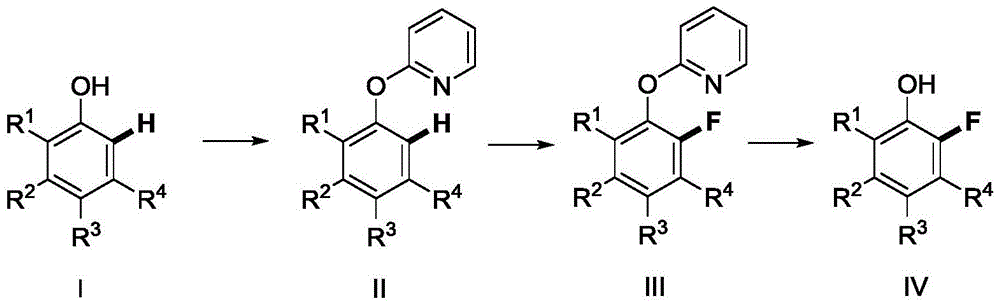
A kind of method of synthesizing 2-fluorophenol compound
A technology of phenolic compounds and fluorophenols, which is applied in the field of synthesizing 2-fluorophenolic compounds, and can solve problems such as unreported phenolic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] [1] 1.57g (10.0mmol) of 2-bromopyridine, 0.10g (0.5mmol) of cuprous iodide, 0.124g (0.1mmol) of pyridine-2-carboxylic acid, 4.240g (20.0mmol) of tripotassium phosphate, 1.128 phenol g (12.0mmol), and 20ml DMSO were added to a 100ml flask. The mixture was heated to 80°C for 24 hours under the protection of nitrogen, and TLC detected that the reaction was complete. Add 50ml of ethyl acetate to dilute, wash and extract, take the organic phase and dry it, remove the solvent under reduced pressure, and the residue is subjected to column chromatography [GF254 silica gel; 100 -200 mesh; Developing agent is V (petroleum ether) / V (ethyl acetate)=10 / 1] separation and purification, collection contains the eluent of product, eluent evaporates solvent and obtains 2-phenoxypyridine 1.368 g (80% yield).
[0052] [2] Add 2-phenoxypyridine (0.855g, 5.0mmol), bis(dibenzylideneacetone)palladium (143.8mg, 0.25mmol), N-fluorobisbenzenesulfonamide into a closed reaction vessel ...
Embodiment 2
[0055]
[0056] [1] 1.57g (10.0mmol) of 2-bromopyridine, 0.10g (0.5mmol) of cuprous iodide, 0.124g (0.1mmol) of pyridine-2-carboxylic acid, 4.240g (20.0mmol) of tripotassium phosphate, 2- Chlorophenol 1.524g (12.0mmol), and 20ml DMSO were added to a 100ml flask. The mixture was heated to 80°C for 24 hours under the protection of nitrogen, and TLC detected that the reaction was complete. Add 50ml of ethyl acetate to dilute, wash and extract, take the organic phase and dry it, remove the solvent under reduced pressure, and the residue is subjected to column chromatography [GF254 silica gel; 100 -200 mesh; Developing agent is V (petroleum ether) / V (ethyl acetate)=10 / 1] separation and purification, collection contains the eluent of product, eluent evaporates solvent and obtains 2-(2-chlorobenzene Oxy)pyridine 1.66 g (81% yield).
[0057] [2] Add 2-(3-chlorophenoxy)pyridine (1.025g, 5.0mmol), bis(dibenzylideneacetone)palladium (143.8mg, 0.25mmol), N-fluoro Bisbenzenesulfonamid...
Embodiment 3
[0060]
[0061][1] 1.57g (10.0mmol) of 2-bromopyridine, 0.10g (0.5mmol) of cuprous iodide, 0.124g (0.1mmol) of pyridine-2-carboxylic acid, 4.240g (20.0mmol) of tripotassium phosphate, 4- Add 1.296g (12.0mmol) of methylphenol and 20ml DMSO into a 100ml flask. The mixture was heated to 80°C for 24 hours under the protection of nitrogen, and TLC detected that the reaction was complete. Add 50ml of ethyl acetate to dilute, wash and extract, take the organic phase and dry it, remove the solvent under reduced pressure, and the residue is subjected to column chromatography [GF254 silica gel; 100 -200 mesh; Developing agent is V (petroleum ether) / V (ethyl acetate)=10 / 1] separation and purification, collection contains the eluent of product, and eluent evaporates solvent to obtain 2-(4-methyl Phenoxy)pyridine 1.54 g (83% yield).
[0062] [2] Add 2-(4-methylphenoxy)pyridine (0.925g, 5.0mmol), bis(dibenzylideneacetone)palladium (143.8mg, 0.25mmol), N- Fluorobisbenzenesulfonamide (1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com